Abstract
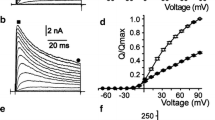
The steady-state effects and rate of action of 4-aminopyridine (4-AP) on normal and chloramine-T (CL-T)-modified voltage-dependent potassium (K) currents were studied in neuroblastoma cells with the whole-cell voltage-clamp current recording technique. 4-AP apparently slows both the activation and inactivation of the normal current but does not modify the time course of the CL-T-modified current. These differential effects of 4-AP are interpreted as resulting from the existence of two types of K channels with different 4-AP sensitivities under normal conditions and similar 4-AP sensitivities after CL-T, which furthermore slows their inactivation [8, 9]. While the onset of 4-AP action on the normal current is delayed and can be described by the difference of two exponentials, the onset of 4-AP action on CL-T-modified current starts immediately after the external application of the drug and can be described by the sum of two exponentials. The 4-AP-induced block of the normal current exhibits use-dependent features and is relieved by long conditioning depolarizations. In contrast, the block of the CL-T-modified current is not use-dependent. At high 4-AP concentrations (1–10 mM), the steady-state block of the normal current reaches a saturating value of 95%, while the steady-state block of the CL-T-modified current and the “unblocked” normal current only reaches a saturating value of 35%. The results suggest that CL-T inhibits a channel or membrane constituent which contributes to the inactivation of channels and increases their apparent affinity for 4-AP when they are in closed or open states. Both the steady-states and rates of the effects of 4-AP can be quantitatively modelled with the assumption of the existence of two receptor sites for 4-AP: a blocking site whose occupancy occludes the channel and an allosteric site whose occupancy inhibits the binding of 4-AP to the blocking site.
Similar content being viewed by others
References
Detoeuf A (1922) Monochlorurée. Préparation de chlorhydrines par action sur les carbures éthyléniques. Bull Soc Chim Fr Mem 31:169–181
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Kirsch GE, Narahashi T (1983) Site of action and active form of aminopyridines in squid axon membranes. J Pharmacol Exp Ther 226:174–179
Kirsch GE, Yeh JZ, Oxford GS (1986) Modulation of aminopyridine block of potassium currents in squid axon. Biophys J 50:637–644
Meves H, Pichon Y (1977) The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol (Lond) 268:511–532
Pongs O (1989) Molecular basis of potassium channel diversity. Pflügers Arch [Suppl] 414:71–75
Quandt FN (1988) Three kinetically distinct potassium channels in mouse neuroblastoma cells. J Physiol (Lond) 395:401–418
Rouzaire-Dubois B, Dubois JM (1989) Chloramine-T-induced modifications of K+ channel inactivation in neuroblastoma cells. Pflügers Arch [Suppl] 414:127–128
Rouzaire-Dubois B, Dubois JM (1990a) Modification of electrophysiological and pharmacological properties of K channels in neuroblastoma cells induced by the oxidant chloramine-T. Pflügers Arch 416:393–397
Rouzaire-Dubois B, Dubois JM (1990b) Tamoxifen blocks both proliferation and voltage-dependent K+ channels of neuroblastoma cells. Cell Signal 2:387–393
Rudy B (1988) Diversity and ubiquity of K channels. Neuroscience 25:729–749
Schechter Y, Burstein Y, Patchornik A (1975) Selective oxidation of methionine residues in proteins. Biochemistry 14:4497–4503
Šimurda J, Simurdova M, Christé G (1989) Use-dependent effects of 4-aminopyridine on transient outward current in dog ventricular muscle. Pflügers Arch 415:244–246
Thompson S (1982) Aminopyridine block of transient potassium current. J Gen Physiol 80:1–18
Ulbricht W, Wagner HH (1976) Block of potassium channels of the nodal membrane by 4-aminopyridine and its partial removal on depolarization. Pflügers Arch 367:77–87
Ulbricht W, Wagner HH, Schmidtmayer J (1982) Effects of aminopyridines on potassium currents of the nodal membrane. In: Lechat P, Thesleff S, Bowman WC (eds) Aminopyridines and similarly acting drugs. (Advances in the biosciences, vol 35) Pergamon, Oxford, pp 29–41
Yeh JZ, Oxford GS, Wu CH, Narahashi T (1976) Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J 16:77–81
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dubois, J.M., Rouzaire-Dubois, B. Interaction of 4-aminopyridine with normal and chloramine-T-modified K channels of neuroblastoma cells. Pflügers Arch. 419, 93–100 (1991). https://doi.org/10.1007/BF00373752
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00373752