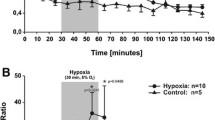
Abstract
A method is described for the measurement and on-line monitoring of muscular extracellular lactate concentration in both anaesthetized and freely moving rats. This method is based on microdialysis sampling and lactic dehydrogenase-catalysed nicotinamide adenine dinucleotide, reduced (NADH)-fluorescence detection techniques. In vivo calibration revealed a resting extracellular lactate concentration of 1.92±0.13 mmol/l (± SEM) in the gastrocnemius muscle of adult male Wistar rats (n=6), while the average whole-blood lactate level was 0.76±0.12 mmol/l (± SEM). This measured extracellular lactate concentration was 1.73-times higher than that deduced from the arterial lactate concentration. Blocking glycolysis with iodoacetate reduced the extracellular lactate concentration to 52±6% (± SEM, n= 4) of the resting level. The extracellular lactate concentration in rat gastrocnemius muscle had increased to significantly (P≤0.05) different levels, 2.4±0.03 (± SEM) or 4.0±0.55 (± SEM) times the control value, 1 h after aortic clamping (n=3) or cardiac arrest (n=3), respectively. Stimulation of the sciatic nerve induced elevations of the extracellular lactate concentration in the tibialis anterior muscle which were linearly related to the recorded isometric force-time integral. We also monitored on-line the changes in extracellular lactate concentration in the tibialis anterior muscle of a swimming rat. Our results indicate that microdialysis lactate reflects also intracellular metabolism. Lactography may be a useful alternative to biopsies and nuclear magnetic resonance spectroscopy in clinical medicine and physiology for the monitoring of metabolism in vivo.
Similar content being viewed by others
References
Alexander GM, Grothusen JR, Schartzman RJ (1988) Flow dependent changes in the effective area of microdialysis probes. Life Sci 43:595–601
Benveniste H, Hansen AJ, Ottoson NS (1989) Determination of brain interstitial concentrations by microdialysis. J Neurochem 52:1741–1750
Dawson MJ (1988) The relation between muscle contraction and metabolism: studies by 31P nuclear magnetic resonance spectroscopy. In: Sugi H, Pollack GH (eds) Molecular mechanism of muscle contraction. Plenum, New York, pp 433–448
Fiaccadori E, Del Canale S, Vitali P, Coffrini E, Ronda N, Guariglia A (1987) Skeletal muscle energetics, acid-base equilibrium and lactate metabolism in patients with severe hypercapnia and hypoxemia. Chest 92:883–887
Harris DH (1986) Movement of oxygen in muscle in skeletal muscle. News Physiol Sci 1:147–149
Harris K, Walker PM, Mickle DAG, Harding R, Gatley R, Wilson GJ, Kuzon B, McKee N, Romaschin AD (1986) Metabolic response of skeletal muscle to ischemia. Am J Physiol 250:H212-H220
Jacobsen I, Sandberg M, Hamberger A (1985) Mass transfer in brain dialysis devices — a new method for the estimation of extracellular amino acids concentration. J Neurosci Methods 15:263–268
Janson PA, Smith U, Lönnroth P (1990) Evidence for lactate production by human adipose tissue in vivo. Diabetologia 33:253–256
Karlsson J (1971) Lactate and phosphagen concentrations in working muscle. Acta Physiol Scand Suppl 358
Kuhr WG, Korf J (1988) Extracellular lactic acid as an indicator of brain metabolism: continuous on-line measurement in conscious, freely moving rats with intrastriatal dialysis. J Cereb Blood Flow Metab 8:130–137
Lindefors N, Amberg G, Ungerstedt U (1989) Intracerebral microdialysis: experimental studies of diffusion kinetics. J Pharmacol Methods 22:141–156
Lönnroth P, Janson PA, Smith U (1987) A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol 253:E228-E231
Madias NE (1986) Lactic acidosis. Kidney Int 29:752–774
Mason MJ, Thomas RC (1988) A micro electrode study of the mechanisms of l-lactate entry into and release from frog sartorius muscle. J Physiol (Lond) 400:459–479
Noy GA, Alberti KGMN (1981) In vivo monitoring of intermediary metabolites. In: Alberti KGMN, Price CP (eds) Advances in clinical biochemistry, vol 2. Churchill Livingstone, Edinburgh, pp 229–241
Parry TJ, Carter TL, McElligot JG (1990) Physical and chemical considerations in the in vitro calibration of microdialysis probes. J Neurosci Methods 32:175–183
Petrofsky JS, Hendershot DM (1984) The interrelationship between blood pressure, intramuscular pressure and isometric endurance in fast and slow twitch skeletal muscle in the cat. Eur J Appl Physiol 53:106–111
Roberts JP, Perry MO, Hariri RJ, Shires GT (1985) Incomplete recovery of muscle cell function following partial but not complete ischemia. Circ Shock 17:253–258
Sahlin K, Edström L, Sjöholm H, Hultman E (1981) Effect of lactic acid accumulation and ATP decrease on muscle tension and relaxation. Am J Physiol 240:C121-C126
Sakawa MN, Petrofsky JS, Phillips CA (1981) Energy cost of submaximal isometric contractions in cat fast and slow twitch muscles. Pflügers Arch 390:164–168
Schasfoort EMC, DeBruin LA, Korf J (1988) Mild stress stimulates rat hippocampal glucose utilization transiently via NMDA receptors, as assessed by lactography. Brain Res 475:58–63
Scheller D, Kolb J (1989) The “internal reference technique” in microdialysis: a practical approach to monitor relative recovery in vivo during normal and pathological conditions. J Neurosci Methods 29:284
Sjogaard G, Saltin B (1982) Extra- and intra-cellular water spaces in muscles of man at rest and with dynamic exercise. Am J Physiol 243:R271-R280
Stainsby WN, Eitzman PD (1988) Roles of CO2, O2, and acid in arteriovenous [H+] difference during muscle contractions. J Appl Physiol 65:1803–1810
Ungerstedt U (1984) Measurement of neurotransmitter release by intracranial dialysis. In: Marsden CA (ed) Measurement of neurotransmitter release in vivo. Wiley, New York, pp 81–107
VanWylen DGL, Willis J, Sodhi J, Weiss RJ, Lasley RD, Mentzer M (1990) Cardiac microdialysis to estimate interstitial adenosine and coronary blood flow. Am J Physiol 258:H1642-H1649
Wakabayashi A, Nakamura Y, Wolley T, Mullin PJ, Watanabe H, Ino T, Connolly JE (1975) Continuous percutaneous monitoring of muscle pH and oxygen pressure. Arch Surg 110:802–805
Westerink BHC, Damsma G, Rollema H, De Vries JB, Horn AS (1987) Scope and limitations of in vivo brain dialysis: A comparison of its application to various neurotransmitter systems. Life Sci 41:1763–1776
Westra HG, De Haan A, Van Doom JE, De Haan EJ (1988) Anaerobic chemical changes and mechanical output during isometric tetany of rat skeletal muscle in situ. Pflügers Arch 412:121–127
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Boer, J., Postema, F., Plijter-Groendijk, H. et al. Continuous monitoring of extracellular lactate concentration by microdialysis lactography for the study of rat muscle metabolism in vivo. Pflügers Arch. 419, 1–6 (1991). https://doi.org/10.1007/BF00373739
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00373739