Abstract
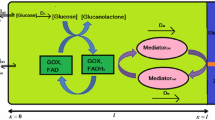
The enzyme glucose oxidase (GO) was covalently immobilized onto a poly(vinyl alcohol) hydrogel, cross-linked with glutardialdehyde and a polyazonium salt. To compare the kinetic parameters of immobilized GO with the known kinetic parameters of soluble GO, the diffusion cell method was used.
Between two compartments, containing solutions with different glucose concentrations, a GO-containing hydrogel membrane was placed. Simultaneous diffusion through and enzymatic reaction in the membrane occurred. In this way diffusional effects of the membrane could be eliminated from the effective kinetic parameters to yield the inherent kinetic parameters.
It appeared that the enzymatic reaction is independent of the oxygen concentration at oxygen concentrations ⩾0.22 mol m−3 (Michaelis constant for oxygen < 0.22 mol m−3). Further, the Michaelis constant for glucose does not change dramatically after immobilizing the enzyme. The maximal reaction rate is depending on the enzyme concentration. As the enzyme concentration in the membrane is not exactly known (mainly due to leakage of enzyme out of the membrane during membrane preparation), only an estimation of the turnover number can be made.
The diffusion cell method is easy to carry out. Still, some recommendations can be made on the performance.
Similar content being viewed by others
Abbreviations
- α g , α 0x :
-
partition coefficient of glucose and oxygen, respectively
- δ :
-
thickness of the wetted membrane (m)
- A m :
-
surface area of membrane (m−2)
- C :
-
constant (mol2 m−3)
- c g , c 0x :
-
concentration of glucose and oxygen, respectively (mol m−3)
- c g,0 c g,δ :
-
glucose concentration at the filter-paper/membrane interface next to compartment A and B, respectively (mol m−3)
- c g, A c g, B :
-
glucose concentration in compartment A and B, respectively (mol m−3)
- c GO :
-
glucose oxidase concentration (mol m−3)
- D eff :
-
effective diffusion coefficient (m2 s−1)
- D m , D sl :
-
diffusion coefficient in, respectively, the membrane and the solution layer (m2 s−1)
- d dl , d df , d sl :
-
thickness of, respectively, the diffusion layer, the filter-paper and the solution layer (m)
- h B :
-
initial slope of concentration versus time curve of compartment B (mol m−3 s−1)
- J :
-
flux (mol m−2 s−1)
- J 0 δ:
-
flux in the membrane at membrane/filter-paper interface next to compartment A and B, respectively (mol m−2 s−1)
- J A , J B :
-
flux leaving compartment A and entering compartment B, respectively (mol m−2 s−1)
- J m :
-
flux through the membrane (mol m−2 s−1)
- k :
-
total mass transfer coefficient (m s−1)
- k 1 , k 2 :
-
rate constant of a particular reaction step (m3 mol−1 s−1)
- k−1, k−2 :
-
rate constant of a particular reaction step (s−1)
- k cat :
-
(intrinsic) catalytic constant of turnover number (s−1)
- k * cat :
-
inherent catalytic constant, determined by inserting D m (s−1)
- k ** cat :
-
inherent catalytic constant, determined by inserting D eff (s−1)
- k m (g) :
-
(intrinsic) Michaelis constant for glucose (mol m−3)
- k m (o) :
-
(intrinsic) Michaelis constant for oxygen (mol m−3)
- k * m (g) :
-
inherent Michaelis constant for glucose (mol m−3)
- k * m (o) :
-
inherent Michaelis constant for oxygen (mol m−3)
- m GO :
-
number of moles of GO present (mol)
- P m :
-
permeability of glucose in the mebrane (m s−1)
- P eff :
-
effective permeability (m s−1)
- V :
-
volume (m3)
- v 0 :
-
initial reaction velocity (mol m−3 s−1)
- V ** max :
-
inherent maximal reaction velocity, determined by inserting Deff (mol m−3 s−1)
- x :
-
distance (m)
References
Carr, P.W.; Bowers, L.D.: Immobilized enzymes in analytical and clinical chemistry, Fundamentals and applications, Chemical analysis, vol. 56, pp. 148–196. New York: John Wiley & Sons, 1980
Kennedy, J.F.; Cabral, J.M.S.: Enzyme immobilization. In: H.-J. Rehm and G. Reed (Eds.): Biotechnology, Enzyme technology, vol. 7a, pp. 347–404. Weinheim, FRG: VCH, 1987
Engasser, J.-M.; Horvath, C.: Diffusion and kinetics with immobilized enzymes. In: L.B. Wingard jr.; E. Katchalski-Katzir and L. Goldstein (Eds.): Applied biochemistry and bioengineering, vol. 1, pp. 127–220. New York: Academic Press, 1976
DeSimone, J.A.; Caplan, S.R.: The determination of local reaction and diffusion parameters of enzyme membranes from global measurements. Biochemistry. 12 (1973) 3032–3038
Sélégny, E.; Broun, G.; Geffroy, J.; Thomas, D.: Méthode de détermination du K m réel d'un enzyme par le régime stationnaire d'une membrane en activité enzymatique. J. Chim. Phys. et Physico-Chimie Biol. 66 (1969) 391–392
Sélégny, E.; Broun, G.; Thomas, D.: Enzymes en phase insoluble. Variation de l'activité enzymatique en fonction de la concentration en substrat. Effets dits ≪ régulateurs ≫ des membranes enzymatiques. C.R. Acad. Sci. Paris 269 (Série D) (1969) 1330–1333
Sélégny, E.; Broun, G.; Thomas, D.: Enzymatically active modelmembranes: experimental illustrations and calculations on the basis of diffusion-reaction kinetics of their functioning, of regulatory effects, of facilitated, retarded and active transports. Physiol. Vég. 9(1) (1971) 25–50
Gibson, Q.H.; Swoboda, B.E.P.; Massey, V.: Kinetics and mechanism of action of glucose oxidase. J. Biol. Chem. 239 (1964) 3927–3934
Walsh, C.: Enzymatic reaction mechanisms. pp. 216–224. San Francisco: W.H. Freeman and Company 1979
van Stroe-Biezen, S.A.M.; Everaerts, F.M.; Janssen, L.J.J.; Tacken, R.A.: Diffusion coefficients of oxygen, hydrogen peroxide and glucose in a hydrogel. Anal. Chim. Acta 273 (1993) 535–560
van Stroe-Biezen, S.A.M.: Heading for a glucose sensor, Designing and testing a new principle, Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, Netherlands (1993)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Stroe-Biezen, S.A.M., van der Loo, J.M.H., Janssen, L.J.J. et al. Determination of the inherent kinetics of immobilized glucose oxidase using a diffusion cell. Bioprocess Engineering 15, 87–94 (1996). https://doi.org/10.1007/BF00372982
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00372982