Abstract
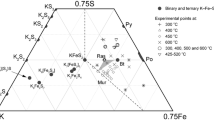
The ternary feldspar system KAlSi3O8 - NaAlSi3O8 - CaAl2Si2O8 was reinvestigated at 650 ° C and 800 ° C (P H2O = 1 kb) using mixtures of crystalline plagioclases and alkali feldspars as starting materials. The compositions of plagioclases and alkali feldspars of the run products were determined by X-ray means. The Or-content of the feldspar phases was determined by measuring the position of the (201) X-ray peak of the unexchanged feldspars, whereas the An-content was determined by measuring the same X-ray peak of the K-exchanged feldspars.
The reaction rate of a reaction leading to a more An-rich plagioclase (type II reaction) is much faster than a reaction producing a more Ab-rich plagioclase (type I). In a type II reaction run times of approximately 20 days are needed to reach new constant plagioclase and alkali feldspar compositions at 650 ° C, and 10 days are needed to reach constant compositions at 800 ° C. In a reaction of type I only the outer zone of the plagioclases reacts to more Abrich compositions. A diffuse zone with a wide range of compositions was observed in 650 ° C runs. Equilibrium could not be reached in these experiments within 45 days. At 800 ° C a new zone having a specific composition develops in 42 days. This new zone is believed to be in equilibrium with the coexisting alkali feldspar. The depth of reaction is calculated as 0.03 μm after 42 days (800 ° C, P f= 1 kb). The reaction between the two feldspar phases could be reversed at 800 ° C. The following compositions are considered to represent equilibrium data at 800 ° C and P t = 1 kb:
-
An 43 Ab 51 Or 6 coexisting with Or 79 Ab 20 An 1, and
-
An 40 Ab 54 Or 6 coexisting with Or 75 Ab 24 An 1.
Recent data obtained with gels of ternary feldspar composition as starting materials do not agree with the results presented in this paper. Gels obviously crystallize spontaneously forming coexisting feldspars of non - equilibrium composition - alkali feldspars too rich in Ab and plagioclases too rich in An.
Similar content being viewed by others
References
Barth, T.E.W.: The feldspar geologic thermometer. N. Jb. Mineral. Abh. 82, 143–154 (1951)
Barth, T.E.W.: The feldspar geologic thermometers. Norsk Geol. Tidsskr. 42, 330–339 (1962)
Barth, T.E.W.: Additional data for the two-feldspar geotermometer. Lithos 1, 305–306 (1968)
Eberhard, E.: Zur Synthese der Plagioklase. Schweiz. Mineral. Petrogr. Mitt. 47, 385–397 (1967)
Iiyama, J.T.: Contribution à l'étude des équilibre sub-solidus du système ternaire orthose-albite-anorthite à l'aide des réactions d''echange d'ions Na-K au contact d' une solution hydrothermale. Bull. Soc. Franc. Mineral. Crist. 89, 442–454 (1966)
Johannes, W.: Melting of plagioclase in the system Ab-An-H2O and Qz-Ab-An-H2O at P H2O = 5 kbars, an equilibrium problem. Contrib. Mineral. Petrol. 66, 295–303 (1978)
Orville, P.M.: Comments on thw two-feldspar geothermometer. Norsk Geol. Tidsskr. 42, 340–346 (1962)
Orivlle, P.M.: Alkali ion exchange between vapor and feldspar phases. Am. J. Sci. 261, 201–237 (1963)
Orivlle, P.M.: Plagioclase cation exchange equilibria with aqueous chloride solution: Results at 700 ° C and 2000 bars in the presence of quartz. Am. J. Sci. 272, 234–272 (1972)
Powell, M., Powell, R.: Plagioclase-alkali-feldspar geothermometry revisited. Mineral. Mag. 41, 253–256 (1977)
Seck, H.A.: Der Einfluß des Drucks auf die Zusammensetzung koexistierender Alkalifeldspäte und Plagioklase im System NaAlSi3O8-KAlSi3O8-CaAl2Si2O8-H2O. Contrib. Mineral. Petrol. 31, 67–86 (1971 a)
Seck, H.A.: Koexistierende Alkalifeldspäte und Plagioklase im System NaAlSi3O8-KAlSi3O8-CaAl2Si2O8-H2O bei Temperaturen von 650 ° C bis 900 ° C. N. Jb. Mineral. Abh. 115, 315–345 (1971 b)
Stornier, Jr., J.C.: A practical two-feldspar geothermometer. Am. Mineralogist 60, 667–674 (1975)
Viswanathan, K.: A new X-ray method to determine the anorthite content and structural state of plagioclases. Contrib. Mineral. Petrol. 30, 332–335 (1971)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Johannes, W. Ternary feldspars: Kinetics and possible equilibria at 800° C. Contr. Mineral. and Petrol. 68, 221–230 (1979). https://doi.org/10.1007/BF00371902
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00371902