Abstract
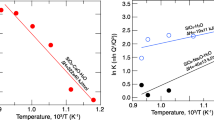
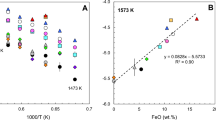
The saturation surfaces of rutile (TiO2), zircon (ZrSiO4), and hafnon (HfSiO4) were determined in anhydrous, peraluminous, high silica liquids of the system SiO2-Al2O3-Na2O-K2O as functions of silica concentration at 1,400° C in air. The saturation concentrations of TiO2, ZrO2, and HfO2 in rutile, zircon, and hafnon-saturated liquids, respectively, decrease smoothly and gradually as functions of increasing silica concentration. Thermodynamic analyses of the data demonstrate that the activity coefficients of TiO2, ZrO2, and HfO2 increase smoothly and gradually as silica concentration is increased from 67 wt-% to 80 wt-%, and that changes in SiO2 of 1 or 2 wt-% result in small changes in the saturation concentrations and activity coefficients of +4 cations. Because the solution behavior of +4 cations in highly siliceous liquids (>75 wt-% SiO2) is predictably different than in less siliceous liquids (70 to 75 wt-% SiO2), classification of highly-siliceous igneous rocks on the basis of silica concentration alone should not be interpreted to mean that their solution chemistry differs significantly from that of less siliceous rocks. The results of this study are compared with other studies of +4 cation solution behavior. From this it is concluded that variations in liquid compositions observed in cogenetic suites of high silica rhyolites cannot cause the observed changes in +4 cation concentrations. Thus, even if a large change in solution behavior of +4 cations is inferred from the large variations in their concentrations, it cannot be due to changes in bulk composition of the parental liquid. In addition, the similarity in the solution behavior of Zr and Hf seen in this study suggests that their solution mechanisms are similar. It is thus unlikely that liquid-state processes can fractionate one with respect to another, and variations in Zr/Hf ratios in suites of extrusive rocks are likely due to crystal-liquid equilibria, e.g., zircon fractionation.
Similar content being viewed by others
References
Bacon CR, MacDonald R, Smith RL, Baedecker PA (1981) Pleistocene high-silica rhyolites of the Coso Volcanic Field, Inyo County, California. JGR, J Geophys Res B86:10223–10241
Cameron KL (1984) Bishop Tuff revisited: new data consistent with crystal fractionation. Science 224:1338–1340
Christiansen EH (1982) The Bishop Tuff revisited: compositional zonation by double-diffusive fractional crystallization. Geol Soc Am Abstr Prog 15:390
Devine JD, Sigurdsson H, Davis AN (1985) Estimates of sulphur and chlorine yield to the atmosphere from volcanic eruptions and potential climatic effects. JGR, J Geophys Res B89:6309–6325
Dickinson JE, Hess PC (1985) Rutile solubility and titanium coordination in silicate liquids. Geochim Cosmochim Acta 49:2289–2296
Douglas RW, Nath P, Paul A (1965) Oxygen ion activity and its influence on the redox equilibrium in glasses. Phys Chem Glasses 6:216–223
Gibbs JW (1948) On the equilibrium of heterogeneous substances. In: The Collected Works of J. Willard Gibbs, Yale Univ Press, New Haven, Connecticut
Harrison TM, Watson EB (1983) Kinetics of zircon dissolution and zirconium diffusion in granitic melts of variable water content. Contrib Mineral Petrol 84:66–72
Hervig RL, Navrotsky A (1984) Thermochemical study of glasses in the system NaAlSi3O8-KAlSi3O8-Si4O8 and the join Na1.6Al1.6Si2.4O8-K1.6Al1.6Si2.4O8. Geochim Cosmochim Acta 48:513–522
Hess PC (1977) Structure of silicate melts. Can Mineral 15:162–178
Hess PC (1986) The role of high field strength cations in silicate melts. In: Advances in Physical Geochemistry, Springer Berlin Heidelberg New York (in press)
Hildreth W (1979) The Bishop Tuff: evidence for the origin of compositional zonation in siliceous magma chambers. Geol Soc Am Spec Paper 180:43–76
Lesher CE (1986) Effects of silicate liquid composition on mineral-liquid element partitioning from Soret diffusion studies. J Geophys Res B91:6123–6141
Mahood GA (1981) Chemical evolution of a Pleistocene rhyolitic center: Sierra La Primavera, Jalisco, Mexico. Contrib Petrol 77:129–149
Mahood G, Hildreth W (1983) Large partition coefficients for trace elements in high-silica rhyolites. Geochim Cosmochim Acta 47:11–30
Michael PJ (1983) Chemical differentiation of the Bishop Tuff and other highsilica magmas through crystallization processes. Geology 11:31–34
Miller CF, Mittlefehldt DW (1982) Depletion of light rareearth elements in felsic magmas. Geology 10:129–133
Miller CF, Mittlefehldt W (1984) Extreme fractionation in felsic magma chambers: a product of liquid-state diffusion or fractional crystallization? Earth Planet Sci Lett 68:151–158
Naski GC, Hess PC (1985) SnO2 solubility: experimental results in peraluminous and peralkaline high silica glasses. EOS: Trans Am Geophys Union 66:412
Nath P, Douglas RW (1965) Cr3+-Cr6+ equilibrium in binary alkali silicate glasses. Phys Chem Glasses 6:197–202
Navrotsky A, Peraudeau P, McMillan P, Coutures J (1982) A thermochemical study of glasses and crystals along the joins silica-calcium aluminate and silica-sodium aluminate. Geochim Cosmochim Acta 46:2039–2047
Navrotsky A, Geisinger KL, McMillan P, Gibbs GV (1985) The tetrahedral framework in glasses and melts — inferences from molecular orbital calculations and implications for structure, thermodnyamics, and physical properties. Phys Chem Minerals, 11:284–298
Paul A, Douglas RW (1965a) Ferrous-ferric equilibrium in alkali silicate glasses. Phys Chem Glasses 6:207–211
Paul A, Douglas RW (1965b) Cerous-ceric equilibrium in binary alkali borate and alkali silicate glasses. Phys Chem Glasses 6:212–215
Rutherford MJ (1967) An experimental determination of iron biotite-alkali feldspar equilibria. J Petrol 10:381–408
Ryerson FJ (1985) Oxide solution mechanisms in silicate melts: systematic variations in the activity coefficients of SiO2. Geochim Cosmochim Acta 49:637–649
Shannon RD (1976) Revised effective ionic radii and systmatic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32:751–767
Watson EB (1979) Zircon saturation in felsic liquids: experimental results and applications to trace element geochemistry. Contrib Mineral Petrol 70:407–419
Watson EB, Harrison TM (1983) Zircon saturation revisited: temperature and composition effects in a variety of crustal magma types. Earth Planet Sci Lett 64:295–304
Watson EB, Ryerson FJ (1986) Rutile saturation in magmas: implications for Nb-Ta-Ti depletion in orogenic magmas. EOS: Trans Am Geophys Union 67:412
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ellison, A.J., Hess, P.C. Solution behavior of +4 cations in high silica melts: petrologic and geochemical implications. Contr. Mineral. and Petrol. 94, 343–351 (1986). https://doi.org/10.1007/BF00371443
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00371443