Abstract
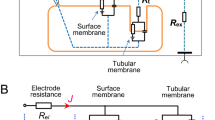
Confluent monolayers of the cultured renal distal tubule cell line (A6) were impaled with microelectrodes under short-circuit conditions. Specific membrane conductances were calculated from equivalent circuit equations. Transport properties of the apical and basolateral membranes were investigated during control conditions and short-term increases in basolateral potassium concentration [K+] from 2.5 to 20 mmol/l, with or without 0.5 mmol/l Ba2+ at the basolateral side. As in most other epithelia, the apical membrane represents the major resistive barrier. Transcellular, apical and basolateral membrane conductances (g c, g o and g i respectively), obtained from 22 acceptable microelectrode studies, averaged 61, 80 and 292 μS/cm2, respectively. There was a highly significant correlation between short-circuit current (I sc) and g o, whereas g i was unrelated to I sc. The I sc, which averaged 4.1 μA/cm2, was almost completely blocked by amiloride. This was associated with fast hyperpolarization; the intracellular potential (V sc) increased from −69 to −83 mV and the fractional apical resistance rose to nearly 100%. Using the values of V sc during amiloride at normal and high [K+], an apparent transference number for K+ at the basolateral membrane of 0.72 can be calculated. This value corresponds with the decrease in g i to about 25% of the control values after blocking the K+ channels with Ba2+. The nature of the remaining conductance is presently unclear. The cellular current decreased during high [K+] and Ba2+, in part resulting from reduction of the electrochemical gradient for apical Na+ uptake due to the depolarization. In addition, g o decreased to less than 40%, which is considerably lower than predicted by the constant-field equation; this might indicate voltage sensitivity of the apical Na+ permeability.
Similar content being viewed by others
References
Armstrong WMcD, Garcia-Diaz JF (1981) Criteria for the use of microelectrodes to measure membrane potentials in epithelia cells. In: Macknight ADC, Leader JP (eds) Epithelial ion and water transport. Raven, New York, pp 43–53
Hamilton KL, Eaton DC (1985) Single-channel recordings from amiloride-sensitive epithelial sodium channel. Am J Physiol 249:C200-C207
Horisberger JD, Giebisch G (1988) Voltage dependence of the basolateral membrane conductance in the amphiuma collecting tubule. J Membr Biol 105:257–263
Koeppen BM, Biagi BA, Giebisch G (1983) Intracellular microelectrode characterization of the rabbit cortical collecting tubule. Am J Physiol 244:F35-F47
Nagel W (1978) The effect of antidiuretic hormone upon electrical potential and resistance of apical and basolateral membranes of frog skin. J Membr Biol 42:99–122
Nagel W (1985) Basolateral membrane ionic conductance in frog skin. Pflügers Arch 405 [Suppl 1]:S39-S43
Nagel W, Van Driessche W (1989) Intracellular potentials of toad urinary bladder. Pflügers Arch 415:121–123
Nagel W, Garcia-Diaz JF, Essig A (1983) Contribution of junctional conductance to the cellular voltage-divider ratio in frog skins. Pflügers Arch 399:336–341
Nagel W, Garcia-Diaz JF, Essig A (1988) Voltage dependence of cellular conductance in frog skin. J Membr Biol 106:13–28
Nelson DJ, Tang JM, Palmer LG (1984) Single channel recordings of apical membrane chloride conductance in A6 epithelial cells. J Membr Biol 80:81–89
Perkins FM, Handler JS (1981) Transport properties of toad kidney epithelium in culture. Am J Physiol 241:C154-C159
Rafferty KA Jr (1969) Mass culture of amphibian cells: methods and observations concerning stability of cell type. In: Mizell M (ed) Biology of amphibian tumors. Springer, Berlin Heidelberg New York, pp 52–81
Sariban-Sohraby S, Latorre R, Burg M, Olans L, Benos D (1984) Amiloride-sensitive epithelial Na+ channels reconstituted into planar lipid bilayer membranes. Nature 308:80–82
Thomas SR, Mintz E (1987) Time-dependent apical membrane K+ and Na+ selectivity in cultured kidney cells. Am J Physiol 253:C1-C6
Ussing HH, Zerahn K (1951) Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand 23:110–127
Walker TC, Fidelman ML, Watlington CO, Biber TUL (1984) Insulin decreases apical cell membrane resistance in cultured kidney cells (A6). Biochem Biophys Res Commun 124:614–618
Yanase M, Handler JS (1986) Adenosine 3′,5′-cyclic monophosphate stimulates chloride secretion in A6 epithelia. Am J Physiol 251:C810-C814
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Granitzer, M., Leal, T., Nagel, W. et al. Apical and basolateral conductance in cultured A6 cells. Pflugers Arch. 417, 463–468 (1991). https://doi.org/10.1007/BF00370940
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00370940