Abstract
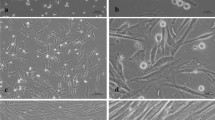
We investigated the increase in mast cell numbers at the sites of inoculation of keratinocyte-derived squamous cell carcinoma cell line (KCMH-1) cells in mice. A significant increase in the number of mast cells was observed at the sites of tumours developed at the sites of inoculation of the KCMH-1 cells. Enhancement of mast cell growth was observed by culturing bone marrow-derived mast cells (BMMC) on NIH/3T3 fibroblast monolayers in the presence of conditioned medium (TCM) obtained from KCMH-1. Activities of known factors for mast cell growth, such as interleukin-3 (IL-3), IL-4, IL-9, IL-10 and stem cell factor (SCF), were not detected in the TCM. Nerve growth factor (NGF) did not induce mast cell growth. Mast cell growth induced by the TCM needed 3T3 fibroblasts. These results suggest that KCMH-1 cells may produce a factor which induces mast cell growth with 3T3 fibroblasts, other than the already known mast cell growth factors. This may be the mechanism of mast cell accumulation at sites of tumours.
Similar content being viewed by others
References
Mikhail GR, Miller-Milinska A (1964) Mast cell population in human skin. J Invest Dermatol 43: 249–254
Claudatus JC Jr, d'Ovidio R, Lospalluti M, Meneghini CL (1986) Skin tumors and reactive cellular infiltrate: Further studies. Acta Derm Venereol (Stockh) 66: 29–34
Ihle JN, Keller J, Oroszlan S, Henderson LE, Copeland TD, Fitch F, Prystowsky MB, Goldwasser E, Schrader JW, Palaszynski E, Dy M, Lebel B (1983) Biological properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, P cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol 131: 282–287
Hamaguchi Y, Kanakura Y, Fujita J, Takeda S, Nakano T, Tarui S, Honjo T, Kitamura Y (1987) Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med 165: 268–273
Hültner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rüde E, Dörmer P, Van Snick J (1990) Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGF III (interleukin 9). Eur J Immunol 20: 1413–1416
Thompson-Snipes L, Dhar V, Bond MW, Mosmann TR, Moore KW, Rennick DM (1991) Interleukin 10: A novel stimulatory factor for mast cells and their progenitors. J Exp Med 173: 507–510
Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu R-Y, Birkett NC, Okino KH, Murdock DC, Jacobsen FW, Langley KE, Smith KA, Takeichi T, Cattanach BM, Galli SJ, Suggs SV (1990) Stem cell factor (SCF) is encorded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 63: 213–224
Zsebo KM, Wypych J, McNiece IK, Lu HS, Smith KA, Karkare SB, Sachdev RK, Yushenkoff VN, Birkett NC, Williams LR; Satyagal VN, Tung W, Bosselman RA, Mendiaz EA, Langley KE (1990) Identification, purification, and biological characterization of hematopoietic stem cell factor from buffalo rat liver-conditioned medium. Cell 63: 195–201
Matsuda H, Kannan Y, Ushio H, Kiso Y, Kanemoto T, Suzuki H, Kitamura Y (1991) Nerve growth factor induces development of connective tissue-type mast cells in vitro from murine bone marrow cells. J Exp Med 174: 7–14
Yung YP, Eger R, Tertian G, Moore MAS (1981) Long-term in vitro culture of murine mast cells. II. Purification of a mast cell growth factor and its dissociation from TCGF. J Immunol 127: 794–799
Prestidge RL, Watson JD, Urdal DL, Mochizuki D, Conlon P, Gillis S (1984) Biochemical comparison of murine colony-stimulating factors secreted by a T cell lymphoma and a myelomonocytic leukemia. J Immunol 133: 293–298
Wong GHW, Clark-Lewis I, McKimm-Breschkin JL, Schrader JW (1982) Interferon-γ-like molecule induces Ia antigens on cultured mast cell progenitors. Proc Natl Acad Sci USA 79: 6989–6993
Levi-Schaffer F, Austen KF, Gravallese PM, Stevens RL (1986) Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci USA 83: 6485–6488
EnerbÄck L (1966) Mast cells in rat gastrointestinal mucosa. II. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand 66: 303–312
Tsuruta Y, Kohashi K, Ohkura Y (1978) Determination of histamine in plasma by high-speed liquid chromatography. J Chromatogr 146: 490–493
Tsuji K, Zsebo KM, Ogawa M (1991) Murine mast cell colony formation supported by IL-3, IL-4, and recombinant rat stem cell factor, ligand for c-kit. J Cell Physiol 148: 362–369
Galli SJ, Wershil BK, Gordon JR, Tsai M, Hammel I (1993) Insights into mast cell development and function derived from analyses of mice carrying mutations at beige, W/c-kit, or Sl/SCF (c-kit ligand) loci. In: Kaliner MA, Metcalfe DD (eds) The mast cell in health and disease. Marcel Dekker, New York Basel Hong Kong, pp 129–202
Fujita J, Nakayama H, Onoue H, Kanakura Y, Nakano T, Asai H, Takeda S, Honjo T, Kitamura Y (1988) Fibroblast-dependent growth of mast cells in vitro: duplication of mast cell depletion in mutant mice W/Wv genotype. J Cell Physiol 134: 78–84
Czarnetzki BM, Wüllenweber I (1988) In vitro migratory response of rat peritoneal macrophages and mast cells towards chemotactic factors and growth factors. J Invest Dermatol 91: 224–227
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakamura, K., Tanaka, T., Morita, E. et al. Enhancement of fibroblast-dependent mast cell growth in mice by a conditioned medium of keratinocyte-derived squamous cell carcinoma cells. Arch Dermatol Res 287, 91–96 (1994). https://doi.org/10.1007/BF00370725
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00370725