Abstract
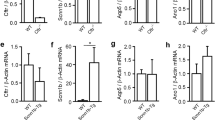
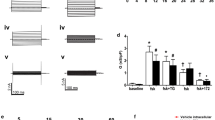
The present study examines the properties of Cl−channels in cultured respiratory cells of cystic fibrosis (CF) patients and normal (N) individuals. In excised membrane patches the conductances for CF and N Cl− channels were larger at positive as compared to negative clamp voltages (V c): 74±2.6 (V c > 0) and 47±2.0 pS (V c < 0) for CF (n= 57) and 69±3.6 (V c > 0) and 45±2.3 pS (V c < 0) for N (n=35). The open probability (P o) of the channel increased markedly with depolarization. Both the voltage dependence of the conductance and of P o contribute to the outward rectification of the channel. The time histogram analysis reveals two open and two closed time constants. The selectivity of the channel was Cl−=Br− =I− > NO −3 ≫ gluconate. The channel was inhibited reversibly by 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB) at 10−7 mol/l to 10−5 mol/l. While Cl− channels were present in cell attached patches of N cells, they were absent in those of CF cells. The mean conductance for cell attached (N) Cl− channels was 76±3.2 pS for positive clamp voltages (V c) and 46±3.9 pS for negative V c (n=8). When the membrane patches were excised from CF cells Cl− currents appeared spontaneously (n=19). The immediate appearance (within 1 s) of Cl− channels after excision was observed at positive (n=6) as well as at negative clamp voltage (n=13). “Excision activation” of CF Cl− channels was observed at low (< 10−9 mol/l) or high (10−3 mol/l) calcium activities on the cytosolic side of the excised patch. Variation of the Ca+ activity (< 10−9–10−3 mol/l) or pH (6.5–8.5) on the cytosolic side exerted no effects on these Cl− channels. These results suggest that Cl− channels are present in the apical membrane of CF and N respiratory cells but they seem to be inhibited in intact CF cells. Excision of the patch and hence removal of the cytosolic “inhibitor” leads to an activation of Cl− channels. The Cl− channels in excised patches of N and CF cells have identical properties.
Similar content being viewed by others
References
Argent BE, Arkle S, Gray MA, Greenwell JR (1987) Two types of calcium-sensitive channels in isolated rat pancreatic duct cells. J Physiol 386:82P
Barthelson RA, Jacoby DB, Widdicombe JH (1987) Regulation of chloride secretion in dog tracheal epithelium by protein kinase C. Am J Physiol 253:C802-C808
Baxter PS, Goldhill J, Hardcastle J, Hardcastle PT, Taylor CJ (1988) Intestinal secretion in cystic fibrosis. Disorders of chloride channel regulation: Cystic fibrosis and secretory diarrhea. Koninklijke Nederlandse Academie van Wetenschappen, Amsterdam
Berschneider HM, Knowles MR, Azizkhan RG, Boucher RC, Tobey NA, Orlando RC, Powell DW (1988) Altered intestinal chloride transport in cystic fibrosis. FASEB J 2:2625–2629
Bijman J, Frömter E (1986) Direct demonstration of high transepithelial chloride-conductance in normal human sweat duct which is absent in cystic fibrosis. Pflügers Arch 407:123–127
Bijman J, Kansen M, Hoogeveen AH, Scholte B, Van der Kamp A, De Jonge H (1988) Electrolyte transport in normal and CF epithelia. In: Wong PYD, Young JA (eds) Exocrine secretion. Hong Kong University Press, pp 17–19
Bijman J, Schölte B, De Jonge HR, Hoogeveen AT, Kansen M, Sinaasappel M, van der Kamp AWM (1988) Chloride transport in cystic fibrosis: chloride channel regulation in cultured sweat duct and cultured nasal epithelium. In: Mastella G, Quinton PM. Cellular and molecular basis of cystic fibrosis, San Francisco Press, San Francisco, pp 133–139
Boucher RC, Larsen EH (1988) Comparison of ion transport by cultured secretory and absorptive canine airway epithelia. Am J Physiol 254:C535-C547
Boucher RC, Knowles MR, Stutts MJ, Gatzy JT (1983) Epithelial dysfunction in cystic fibrosis lung disease. Lung 161:1–17
Candia OA, Montoreano R, Podos SM (1977) Effects of the ionophore A23187 on chloride transport across isolated frog cornea. Am J Physiol 233:F94-F101
Cartwright CA, McRoberts JA, Mandel KG, Dharmsathaphorn K (1985) Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest 76:1837–1842
Chase H, Wong S (1988) Simultaneous measurements of intracellular free [Ca] and Cl secretion in T84 cells reveal Ca's role in the action of carbachol. J Gen Physiol 42:25a
Colquhoun D, Hawkes AG (1983) The principles of the stochastic interpretation of ion channel mechanism. In: Sakmann B, Neher E (eds) Single channel recording. Plenum Press, New York, pp 135–175
Dreinhöfer J, Gögelein H, Greger R (1988) Blocking kinetics of Cl− channels in colonic carcinoma cells (HT29) as revealed by 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB). Biochim Biophys Acta 956:135–142
Evans MG, Marty A, Tan YP, Trautmann A (1986) Blockage of Ca-activated Cl conductance by furosemide in rat lacrimal glands. Pflügers Arch 406:65–68
Findlay I, Petersen OH (1985) Acetylcholine stimulates a Ca2+-dependent Cl− conductance in mouse lacrimal acinar cells. Pflügers Arch 403:328–330
Frizzell RA (1987) Cystic fibrosis: a disease of ion channels? TINS 10:190–193
Frizzell RA, Field M, Schultz SG (1979) Sodium-coupled chloride transport by epithelial tissues. Am J Physiol 236:F1-F8
Frizzell RA, Rechkammer GR, Shoemaker RL (1986) Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science 233:558–560
Frizzell RA, Schoumacher RA, Shoemaker RL, Halm DR (1987) Chloride channel regulation in secretory epithelial cells. Pediatr Pulmonol 1 (Suppl):24–25
Frömter E, Disser J (1988) Properties of Na+ channels and Cl−channels in sweat duct and nasal polyp epithelia. Disorders of chloride channel regulation: Cystic fibrosis and secretory diarrhea. Koninklijke Nederlandse Academie van Wetenschappen, Amsterdam
Fulford GR, Blake JR (1986) Mucociliary transport in the lung. J Theor Biol 121:381–402
Gögelein H (1988) Chloride channels in epithelia. Biochim Biophys Acta 947:521–547
Gögelein H, Greger R (1984) Single channel recordings from the basolateral and apical membranes of renal proximal tubules. Pflügers Arch 401:424–426
Gögelein H, Greger R (1986) A voltage-dependent ionic channel in the basolateral membrane of late proximal tubules of the rabbit kidney. Pflügers Arch 407:142–148
Gögelein H, Greger R, Schlatter E (1987) Potassium channels in the basolateral membrane of the rectal gland of squalus acanthias. Regulation and inhibitors. Pflügers Arch 409:107–113
Goldstein JL, Nash NT, Al-Bazzaz F, Layden TJ, Rao MC (1988) Rectum has abnormal ion transport but normal cAMP-binding proteins in cystic fibrosis. Am J Physiol 254:C719-C724
Greger R, Kunzelmann K (1988) Chloride channels. Disorders of chloride channel regulation: Cystic fibrosis and secretory diarrhea. Koninklijke Nederlandse Academie van Wetenschappen, Amsterdam
Greger R, Schlatter E (1984) Mechanism of NaCl secretion in rectal gland tubules of spiny dogfish (Squalus acanthias). I. Experiments in isolated in vitro perfused rectal gland tubules. Pflügers Arch 402:63–75
Greger R, Schlatter E (1984) Mechanism of NaCl secretion in rectal gland tubules of spiny dogfish (Squalus acanthias). II. Effects of inhibitors. Pflügers Arch 402:364–375
Greger R, Gögelein H, Schlatter E (1987) Potassium channels in the basolateral membrane of the rectal gland of the dogfish (Squalus acanthias). Pflügers Arch 409:100–106
Greger R, Schlatter E, Gögelein H (1987) Chloride channels in the luminal membrane of the rectal gland of the dogfish (Squalus acanthias). Properties of the “larger” conductance channel. Pflügers Arch 409:114–121
Halm DR, Rechkemmer GR, Schoumacher RA, Frizzell RA (1988) Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol 254:C505-C 511
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hayslett JP, Gögelein H, Kunzelmann K, Greger R (1987) Characteristics of apical chloride channels in human colon cells (HT29). Pflügers Arch 410:487–494
Knowles MR, Gatzy JT, Boucher RC (1983) Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest 71:1410–1417
Kunzelmann K, Ünal Ö, Beck C, Emmrich P, Arndt HJ, Greger R (1988) Ion channels of cultured respiratory epithelial cells of patients with cystic fibrosis. Pflügers Arch 411:R68
Kunzelmann K, Pavenstädt H, Beck C, Ünal Ö, Emmrich P, Arndt HJ, Greger R (1989) Characterization of potassium channels in respiratory cells. I. General properties. Pflügers Arch 414:291–296
Kunzelmann K, Pavenstädt H, Greger R (1989) Characterization of potassium channels in respiratory cells. II. Inhibitors and Regulation. Pflügers Arch 414:297–303
Li M, McCann JD, Liedtke CM, Nairn AC, Greengard P, Welsh MJ (1988) Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature 331:358–360
Marin MG, Zaremba MM (1978) Effect of calcium on ion transport and electrical properties of tracheal epithelium. J Appl Physiol 44:900–904
Miller C, White MH (1984) Dimeric structure of single chloride channels from Torpedo electroplax. Proc Natl Acad Sci USA 81:2772–2775
Olver RE, Davis B, Marin MG, Nadel JA (1975) Active transport of Na+ and Cl− across the canine tracheal epithelium in vitro. Am Rev Respir Dis 112:811–815
Orlando RC, Powell DW, Boucher RC, Knowles MR (1985) Colonic and esophageal potential difference measurements in cystic fibrosis. Gastroenterology 88:1524
Quinton PM (1983) Chloride impermeability in cystic fibrosis. Nature 301:421–422
Quinton PM (1986) Missing Cl conductance in cystic fibrosis. Am J Physiol 251:C649-C652
Rohlicek V, Fröbe U, Gögelein H, Greger R (1989) Versatile supplement device with remote control for the control of patch clamp experiments. Pflügers Arch 413:444–446
Sakmann B, Neher E (1984) Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol 46:455–472
Sato K, Sato F (1981) Role of calcium in cholinergic and adrenergic mechanisms of eccrine sweat secretion. Am J Physiol 241:C113-C120
Schoumacher RA, Shoemaker RL, Halm DR, Tallant EA, Wallace RW, Frizzell RA (1987) Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature 330:752–754
Smith PL, Welch MJ, Stoff JS, Frizzell RA (1982) Chloride secretion by canine tracheal epithelium. I. Role of intracellular cAMP levels. J Membr Biol 70:217–226
Wangemann P, Di Stefano A, Wittner M, Englert HC, Lang HJ, Schlatter E, Greger R (1986) Cl−-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflügers Arch 407:S128-S141
Welsh MJ (1986) An apical-membrane chloride channel in human tracheal epithelium. Science 232:1648–1649
Welsh MJ (1987) Electrolyte transport by airway epithelia. Physiol Rev 67:1143–1184
Welsh MJ (1987) Effect of phorbol ester and calcium ionophore on chloride secretion in canine tracheal epithelium. Am J Physiol 253:C828-C834
Welsh MJ, Liedtke CM (1986) Chloride and potassium channels in cystic fibrosis airway epithelia. Nature 322:467–470
Welsh MJ, Mc Cann JD, Dearborn DG (1987) Chloride and sodium channel currents in normal and cystic fibrosis sweat duct epithelium. Fed Proc 46:5567
Widdicombe JH (1986) Cystic fibrosis and β-adrenergic response of airway epithelial cell cultures. Am J Physiol 251:R818-R822
Widdicombe JH, Welsh MJ, Finkbeiner WE (1985) Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc Natl Acad Sci USA 82:6167–6171
Yankaskas JR, Cooton CU, Knowles MR, Gatzy JT (1985) Culture of human nasal epithelium cells on collagen matrix supports. Am Rev Respir Dis 132:1281–1287
Yankaskas JR, Gatzy JT, Knowles MR, Boucher RC (1985) Persistence of abnormal chloride ion permeability in cystic fibrosis nasal epithelial cells in heterologous culture. Lancet 1:954–956
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kunzelmann, K., Pavenstädt, H. & Greger, R. Properties and regulation of chloride channels in cystic fibrosis and normal airway cells. Pflügers Arch 415, 172–182 (1989). https://doi.org/10.1007/BF00370589
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00370589