Abstract
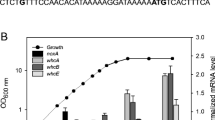
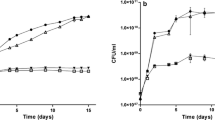
The putative products of six Azotobacter vinelandii chromosomal open reading frames (ORFs) were suggested to be involved in dihydrogen (H2) metabolism [Chen and Mortenson (1992) Biochim Biophys Acta 1131, 199–202]. A promoterless lacZ-containing cassette was used to disrupt the ORFs. Qualitative analysis revealed that the lacZ genes were expressed only in those mutants where the directions of the inserted lacZ were identical to those of the ORFs, showing that the six ORFs were transcribed as predicted. Unlike wildtype (w.t.), none of the mutants could perform dioxygen (O2)-dependent H2-oxidation, even though Western immunoanalyses showed that the hydrogenase large subunit was present although in amounts less than in w.t. Only one of the mutants (a hypB mutant), grown in nickel-enriched media, showed meaningful restoration of the H2-oxidizing ability. From the above observations it is concluded that (a) the six-ORF region is transcriptionally active and involved in H2-oxidation, (b) the product of hypB is needed for nickel activation of hydrogenase, and (c) the six ORFs (genes) belong to two or more operons. Possible roles of the gene products for the assembly, modification, and processing of hydrogenase from its apoproteins and metal centers are discussed.
Similar content being viewed by others
Literature Cited
Adams MWW, Mortenson LE, Chen J-S (1985) Hydrogenase. Biochim Biophys Acta 594:105–176
Birkeet CR, Foster KE, Johnson L, Gull K (1985) Use of monoclonal antibodies to analyse the expression of a multitubulin family. FEBS Lett 187:211–218
Bush JA, Wilson PW (1959) A non-gummy chromogenic strain of Azotobacter vinelandii. Nature 184:381
Chen JC, Mortenson LE (1992a) Two open reading frames (ORFs) identified near the hydrogenase structural genes in Azotobacter vinelandii, the first ORF may encode for a polypeptide similar to rubredoxins. Biochim Biophys Acta 1131:122–124
Chen JC, Mortenson LE (1992b) Identification of six open reading frames from a region of the Azotobacter vinelandii genome likely involved in dihydrogen metabolism. Biochim Biophys Acta 1131:199–202
Colbeau A, Richaud P, Toussaint B, Caballero FJ, Elster C, Delphin C, Smith RL, Chabert J, Vignais PM (1993) Organization of the genes necessary for hydrogenase expression in Rhodobacter capsulatus. Mol Microbiol 8:15–29
Darbre A (1986) Biuret method for samples containing thiols. In: Practical protein chemistry—a handbook. New York: John Wiley & Sons, pp 292–293
Dernedde J, Eitinger M, Friedrich B (1993) Analysis of a pleiotropic gene region involved in formation of catalytically active hydrogenases in Alcaligenes eutrophus H16. Arch Microbiol 159:545–553
Du L, Tibelius KH (1994) The hupB gene of the Azotobacter chroococcum hydrogenase gene cluster is involved in nickel metabolism. Curr Microbiol 28:21–24
Eitinger T, Friedrich B (1991) Cloning, nucleotide sequence, and heterologous expression of a high-affinity nickel transport gene from Alcaligenes eutrophus. J Biol Chem 266:3222–3227
Emerich DW, Ruiz-Argueso T, Ching TM, Evans HJ (1979) Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol 137:153–160
Garg RP, Menon AL, Jacobs K, Robson RM, Robson RL (1994) The hypE gene completes the gene cluster for H2-oxidation in Azotobacter vinelandii. J Mol Biol 236:390–396
Jacobi A, Rossmann R, Bock A (1992) The hyp operon gene products are required for the maturation of catalytically active hydrogenase isoenzymes in Escherichia coli. Arch Microbiol 158:444–451
Kokotek W, Lotz W (1989) Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene 84:467–471
Kovacs KL, Seefeldt LC, Tigyi G, Doyle CM, Mortenson LE, Arp DJ (1989) Immunologica relationship among hydrogenases. J Bacteriol 171:430–435
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lutz S, Jacobi A, Schlensog V, Bohm R, Sawers G, Bock A (1991) Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol 5:123–135
Maier T, Jacobi A, Sauter M, Bock A (1993) The product of the hypB gene, which is required for nickel incorporation into hydrogenases, is a novel guanine nucleotide-binding protein. J Bacteriol 175:630–635
Maniatis T, Fritsch EF, Sambrook J (1982) Southern transfer. In: Molecular Cloning. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, pp 382–385
Menon AL, Stults LW, Robson RL, Mortenson LE (1990) Cloning, sequencing and characterization of the [NiFe]hydrogenase-encoding structural genes (hoxK and hoxG) from Azotobacter vinelandii. Gene 96:67–74
Menon AL, Mortenson LE, Robson RL (1992) Nucleotide sequences and genetic analysis of hydrogen oxidation (hox) genes in Azotobacter vinelandii. J Bacteriol 174:4549–4557
Navarro C, Wu LF, Mandrand-Berthelot M-A (1993) The nik operon of Escherichia coli encodes a periplasmic binding-protein-dependent transport system for nickel. Mol Microbiol 9:1181–1191
Page WJ (1985) Genetic transformation of molybdenum-starved Azotobacter vinelandii: increased transformation frequency and recipient range. Can J Microbiol 31:659–662
Rey L, Murillo J, Hernando Y, Hidalgo E, Cabrera E, Imperial J, Ruiz-Argueso T (1993) Molecular analysis of a microaerobically induced operon required for hydrogenase synthesis in Rhizobium leguminosarum biovar viciae. Mol Microbiol 8:471–481
Robson RL, Chesshyre JA, Wheeler C, Jones R, Woodley PR, Posgate JR (1984) Genome size and complexity in Azotobacter chroococcum. J Gen Microbiol 130:1603–1612
Sayavedra-Soto LA, Arp DJ (1992) The hoxZ gene of the Azotobacter vinelandii hydrogenase operon is required for activation of hydrogenase. J Bacteriol 174:5295–5301
Seefeldt LC, Arp DJ (1986) Purification to homogeneity of Azotobacter vinelandii hydrogenase: a nickel and iron containing αβ dimer. Biochimie 68:25–34
Strandberg GW, Wilson PW (1968) Formation of the nitrogenfixing enzyme system in Azotobacter vinelandii. Can J Microbiol 14:25–31
Sweet WJ, Houchins JP, Rosen PR, Arp DJ (1980) Polarographic measurement of H2 in aqueous solutions. Anal Biochem 107:337–340
Tibelius KH, Du L, Tito D, Stejskal F (1993) The Azotobacter chroococcum hydrogenase gene cluster: sequences and genetic analysis of four accessory genes, hupA, hupB, hupY and hupC. Gene 127:53–61
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Xu H-W, Wall JD (1991) Clustering of genes necessary for hydrogen oxidation in Rhodobacter capsulatus. J Bacteriol 173:2401–2405
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp 18 and pUC19 vectors. Gene 33:103–119
Yates MG, Campbell FO (1989) The effect of nutrient limitation on the competition between an H2-uptake hydrogenase positive (Hup+) recombinant strain of Azotobacter chroococcum and the Hup- mutant parent in mixed populations. J Gen Microbiol 135:221–226
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, J.C., Mortenson, L.E. & Seefeldt, L.C. Analysis of a gene region required for dihydrogen oxidation in Azotobacter vinelandii . Current Microbiology 30, 351–355 (1995). https://doi.org/10.1007/BF00369862
Issue Date:
DOI: https://doi.org/10.1007/BF00369862