Abstract
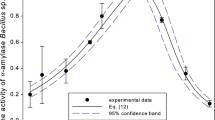
A mathematical model is described for the simultaneous saccharification and ethanol fermentation (SSF) of sago starch using amyloglucosidase (AMG) and Zymomonas mobilis. By introducing the degree of polymerization (DP) of oligosaccharides produced from sago starch treated with α-amylase, a series of Michaelis-Menten equations were obtained. After determining kinetic parameters from the results of simple experiments carried out at various substrate and enzyme concentrations and from the subsite mapping theory, this model was adapted to simulate the SSF process. The results of simulation for SSF are in good agreement with experimental results.
Similar content being viewed by others
Abbreviations
- α g/g:
-
rate coefficient of production
- μ max 1/h:
-
maximum specific growth rate
- E %, v/w:
-
AMG concentration
- G 1 mmol/l:
-
glucose concentration
- G c mmol/l:
-
glucose concentration consumed
- G f mmol/l:
-
glucose concentration formed
- G n mmol/l:
-
n-mer maltooligosaccharide concentration
- K i g/l:
-
ethanol inhibition constant for ethanol production
- K g mmol/l:
-
glucose inhibition constant for glucose production
- K p mmol/l:
-
glucose limitation constant for ethanol production
- K x mmol/l:
-
glucose limitation constant for cell growth
- K m,n mmol/l:
-
Michaelis-Menten constant for n-mer oligosaccharide
- k e %, v/w:
-
enzyme limitation constant
- k es :
-
proportional constant
- k max, n 1/s:
-
maximal velocity for n-mer digestion
- k s g/l:
-
substrate limitation constant
- m s g/g:
-
maintenance energy
- MW n g/mol:
-
molecular weight of n-mer oligosaccharide
- P g/l:
-
ethanol concentration
- P 0 g/l:
-
initial ethanol concentration
- P m g/l:
-
maximal ethanol concentration
- Q pm g/(g · h):
-
maximum specific ethanol production rate
- S n mmol/h:
-
branched n-mer oligosaccharide concentration
- S 0 g/l:
-
initial starch concentration
- S sta g/l:
-
starch concentration
- S tot g/l:
-
total sugar concentration
- V max, n 1/h:
-
maximum digestion rate of n-mer oligosaccharide
- V 0 g/(l · h):
-
initial glucose formation rate
- X g/l:
-
cell mass
- X 0 g/l:
-
initial cell mass
- Y p/s g/g:
-
ethanol yield
- Y x/s g/g:
-
cell mass yield
References
Lee, J. H.; Pagan, R. J.; Rogers, P. L.: Continuous simultaneous saccharification and fermentation of starch using Zymomonas mobilis. Biotechnol. Bioeng. 25 (1983) 659–669
de Menezes, T. J. B.: Starchy materials for alcohol fuel production. Process Biochem. 17 (1982) 32–35
Poosaran, N.; Heyes, R. H.; Rogers, P. L.: Ethanol production from cassava starch using a highly productive strain of Zymomonas mobilis and Saccharomyces uvarum ATCC 26602. Biomass. 7 (1985) 171–183
Pazur, J. H.; Ando, T.: The hydrolysis of glucosyl oligosaccharides with α-D-(1→4) and α-D-(1→6) bonds by fungal amyloglucosidase. J. Biol. Chem. 235 (1960) 297–302
Norman, B. E.: The application of polysaccharide degrading enzymes in the starch industry. In: Berkeley, R. C. W.; Gooday, G. W.; Ellwood, D. C. (Eds.): Microbial Polysaccharides and Polysaccharases, pp. 339–376. New York: Academic Press 1979
Ghosh, P.; Pamment, N. B.; Martin, W. R. B.: Simultaneous saccharification and fermentation of cellulose: Effect of β-D-glucosidase activity and ethanol inhibition of cellulases. Enzyme Microb. Technol. 4 (1982) 425–430
Asenjo, J. A.: Modelling the bioconversion of cellulose into microbial products: Rate limitations. Process Biochem. 19 (1984) 217–224
Lee, G. M.; Kim, C. H.; Zainal, A.; Han, M. H.; Rhee, S. K.: Sago starch saccharification and simultaneous ethanol fermentation by amyloglucosidase and Zymomonas mobilis. J. Chem. Tech. Biotechnol. 38 (1987) 235–242
Pazur, J. H.; Kleppe, K.: The hydrolysis of α-D-glucosides by amyloglucosidase from Aspergillus niger. J. Biol. Chem. 237 (1984) 1002–1006
Hiromi, K.: Interpretation of dependency of rate parameters on the degree of polymerization of substrate in enzyme-catalyzed reactions: Evaluation of subsite affinities of exo-enzyme. Biochem. Biophys. Res. Commun. 40 (1970) 1–6
Hiromi, K.; Nitta, Y.; Numata, C.; Ono, S.: Subsite affinities of glucoamylase: Examination of the validity of the subsite theory. Biochim. Biophys. Acta 302 (1973) 362–375
Tanaka, A.; Fukuchi, Y.; Ohnishi, M.; Hiromi, K.; Aibara, S.; Morita, Y.: Fractionation of isozymes and determination of the subsite structure of glucoamylase from Rhizopus niveus. Agr. Biol. Chem. 47 (1983) 573–580
Kim, C. H.; Lee, G. M.; Zainal, A.; Han, M. H.; Rhee, S. K.: Immobilization of Zymomonas mobilis and amyloglucosidase for ethanol production from sago starch. Enzyme Microb. Technol. 10 (1988) 426–430
Ono, S.; Hiromi, K.; Zinbo, M.: Kinetic studies on gluc-amylase. I. The influence of chain length of linear substrates on the rate parameters. J. Biochem. 55 (1964) 315–320
Hiromi, K.; Hamauzu, Z.; Takahashi, K.; Ono, S.: Kinetic studies on gluc-amylase. II. Competition between two types of substrate having α-1,4 and α-1,6 glucosidic linkage. J. Biochem. 59 (1966) 411–418
Hiromi, K.; Takahashi, K.; Hamauzu, Z.; Ono, S.: Kinetic studies on gluc-amylase. III. The influence of pH on the rates of hydrolysis of maltose and panose. J. Biochem. 59 (1966) 469–475
Hiromi, K.; Kawai, M.; Ono, S.: Kinetic studies on gluc-amylase. IV. Hydrolysis of isomaltose. J. Biochem. 59 (1966) 476–480
Abduliah, M.; Fleming, I. D.; Taylor, P. M.; Whelan, W. J.: Substrate specificity of the amyloglucosidase of Aspergillus niger. Biochem. J. 89 (1963) 359–369
Kuo, S. S.: In: Computer applications of numerical methods. Massachusetts: Addison-Wesley Publishing Company, Inc. 1972
Levenspiel, O.: The Monod equation: A revisity and a generalization to product inhibition situations. Biotechnol. Bioeng. 22 (1980) 1671–1687
Ghose, T. K.; Tyagi, R. D.: Rapid ethanol fermentation of cellulose hydrolysate. II. Product and substrate inhibition and optimization of fermentor design. Biotechnol. Bioeng. 21 (1979) 1401–1420
Bazua, C. D.; Wilke, C. R. P.: Ethanol effects on the kinetics of a continuous fermentation with Saccharomyces cerevisiae. Biotechnol. Bioeng. Symp. No. 7 (1977) 105–118
Leudeking, R.; Piret, E. L.: A kinetic study of the lactic acid fermentation: Batch process at controlled pH. J. Biochem. Microbiol. Technol. Eng. 1 (1959) 393–412
Rogers, P. L.; Lee, K. J.; Tribe, D. E.: Kinetics of alcohol production by Zymomonas mobilis at high sugar concentrations. Biotechnol. Lett. 1 (1979) 165–170
Tan, K.: Sago production in southwest peninsular Malaysia. In: Stanton, W. R.; Flach, M. (Eds.): Proceedings of the Second International Sago Symposium, pp. 56–83. The Hague/Boston/London: Martinus Nijhoff Publishers 1980
Rhee, S. K.; Lee, G. M.; Han, Y. T.; Zainal, A.; Han, M. H.; Lee, K. J.: Ethanol production from cassava and sago starch using Zymomonas mobilis. Biotechnol. Lett. 6 (1984) 615–620
Novo Industri Enzyme Information. File Number A5170-GB (1972)
Lee, K. J.; Rogers, P. L.: The fermentation kinetics of ethanol production by Zymomonas mobilis. Chem. Eng. J. 27 (1983) 831–838
Hyung, S. Y.; Chen, J. C.: Analysis of the kinetics of ethanol fermentation with Zymomonas mobilis considering temperature effect. Enzyme Microb. Technol. 10 (1988) 431–439
Jobses, I. M. L.; Roels, J. A.: The inhibition of the maximum specific growth and fermentation rate of Zymomonas mobilis by ethanol. Biotechnol. Bioeng. 28 (1986) 554–563
Jobses, I. M. L.; Egberts, G. T. C.; Luyben, K. C. A. M.; Roels, J. A.: Fermentation kinetics of Zymomonas mobilis at high ethanol concentrations: Oscillations in continuous cultures. Biotechnol. Bioeng. 28 (1986) 868–877
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, C.G., Kim, C.H. & Rhee, S.K. A kinetic model and simulation of starch saccharification and simultaneous ethanol fermentation by amyloglucosidase and Zymomonas mobilis. Bioprocess Engineering 7, 335–341 (1992). https://doi.org/10.1007/BF00369488
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00369488