Abstract
Bleomycin produces pulmonary fibrosis in mice when given as a single intratracheal instillation or as multiple subcutaneous injections. Both models are associated with a significant deposition of collagen in the lungs but differ in the location of the initial lesions. Intratracheal instillation of bleomycin directs lesions to peribronchial or peribronchiolar sites, whereas subcutaneous injection produces lesions in subpleural and perivascular locations. It was therefore of interest to analyse the bronchoalveolar lavage (BAL) fluid for differences in the cellular composition, especially of intraepithelial T lymphocytes characterised by the expression of the integrin αEβ7.
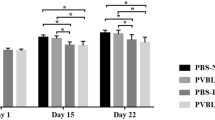
Intracheal instillation of bleomycin induced a 5 to 6 times higher number of neutrophils and lymphocytes in BAL fluid than the subcutaneous model. Furthermore, intratracheal instillation of bleomycin induced the infiltration of eosinophilic neutrophils, a peculiar subtype of neutrophils often found in rodents, which were not found in BAL after subcutaneous injection of bleomycin. In both models of bleomycin injection, however, CD4+, CD8+, NK, and γδ T lymphocytes were detected with dominance of the CD4+ T cell population. Analysis of the expression of the integrin αEβ7 revealed similar numbers of αEβ7+ cells in the CD4+ and γδ T cell populations in both models but significantly lower numbers of αEβ7+ T cells were found in the BAL fluid within the CD8+ T cell population after subcutaneous injection of bleomycin compared to intratracheal instillation.
These results show that a difference in route of bleomycin administration not only causes changes in the localisation of the lesions but that this variation may be reflected in alterations within the BAL leucocyte population especially within the intraepithelial T lymphocytes.
Similar content being viewed by others
References
Adamson IYR, Bowden DH (1974) The pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Pathol 77:185–197
Adamson IYR, Bowden DH (1983) Endothelial injury and repair in radiation-induced pulmonary fibrosis. Am J Pahtol 112:224–230
Anonymous (1995) Guiding principles in the use of animals in toxicology. Toxicol Appl Pharmacol 130:180
Aso Y, Yoneda K, Kikkawa Y (1976) Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Lab Invest 35:558–568
Austrup F, Rebstock S, Kilshaw PJ et al. (1995) Transforming growth factor-β 1-induced expression of the mucosa-related integrin αE on lymphocytes is not associated with mucosa-specific homing. Eur J Immunol 25:1487–1491
Boismenu R, Havran WL (1994) Modulation of epithelial cell growth by intraepithelial γδ T cells. Science 266:1253–1255
Bonikos DS, Bensch KG, Northway WH (1976) Oxygen toxicity in the newborn: the effect of chronic continuous 100% oxygen exposure on the lungs of newborn mice. Am J Pathol 85:623–650
Braun RK, Sterner-Kock A, Kilshaw PJ, Ferrick DA, Giri SN (1996) Integrin αEβ7 expression on BAL CD4+, CD8+, and γδ T-cells in the bleomycin-induced lung fibrosis in mouse. Eur Respir J 9:673–679
Cepek KL, Parker CM, Madara JL et al. (1993) Integrin αEβ7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol 150:3459–3470
Cerf-Bensussan N, Jarry A, Brousse N et al. (1987) A monoclonal antibody (HML-1) defining a novel membrane molecule on human intestinal lymphocytes. Eur J Immunol 17:1279–1285
Chandler DB, hyde DM, Giri SN (1983) morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am J Pathol 112:170–177
Chang LY, Overby LH, Brody AR et al. (1988) Progressive lung cell reactions and extracellular matrix production after a brief exposure to asbestos. Am J Pathol 131:156–170
Chao C-C, Sandor M, Dailey M (1994) Expression and regulation of adhesion molecules by γδ T cells from lymphoid tissues and intestinal epithelium. Eur J Immunol 24:3180–3187
Clark JG, Kuhn C (1982) Bleomycin-induced pulmonary fibrosis in hamsters: effects of neutrophil-depletion in lung collagen synthesis. Am Rev Respir Dis 126:737–739
Constant P, Davodeau F, Peyrat M-A et al. (1994) Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 264:267–270
Crooke ST, Bradner WT (1976) Bleomycin, a review. J Med 7:249–330
Fasske E, Morgenroth K (1983) Experimental bleomycin lung in mice. A contribution to the pathogenesis of pulmonary fibrosis. Lung 161:133–146
Ferrick DA, Schrenzel MD, Mulvania T et al. (1995) Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2- stimulating pathogens by γδ T cells in vivo. Nature 373:255–257
Giri SN, Hollinger MA, Schiedt MJ (1981) The effects of paraquat and superoxide dismutase on pulmonary vascular permeability and edema in mice. Arch Environ Health 36:149–154
Giri SN (1990) Pharmacologic perspectives in pulmonary fibrosis research. In: Hollinger MA (ed) Focus on pulmonary pharmacology and toxicology. CRC Press, Boca Raton, Florida, pp 19–55
Grau GE, Lambert PH, Vassalli P et al. (1989) Tumor necrosis factor (TNF) and pathology; its relationships with other cytokines. Schweiz Med Wochenschr 119:1756–1761
Harrison JH, Lazo JS (1987) High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther 243:1185–1194
Hawkey CM, Denett TB (1989) Color atlas of comparative veterinary hematology: normal and abnormal blood cells in mammals, birds, and reptiles. Wolfe Medical Publications Ltd. London
Hsieh B, Schrenzel MD, Mulvania T et al. (1996) In vivo cytokine production in murine usterosis. Evidence for immunoregulation by γδ+ T cells. J Immunol 156:232–237
Janick-Buckner D, Ranges GE, Hacker MP (1989a) Alterations of bronchoalveolar lavage cell populations following bleomycin treatment in mice. Toxicol Appl Pharmacol 100:465–473
Janick-Buckner D, Ranges GE, Hacker MP (1989b) Effect of cytotoxic monoclonal antibody depletion of T-lymphocyte sub-populations on bleomycin induced lung damage in C57BL/6J mice. Toxicol Appl Pharmacol 100:474–484
Karecla PI, Bowden SJ, Green SJ, et al. (1995) Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin αM290β7 (αEβ7). Eur J Immunol 25:852–856
Karpel JP, Aldrich TK, Mitsudo S et al. (1989) Lung lymphocytes in bleomycin-induced pulmonary disease. Lung 167:163–172
Kehrer JP, Witschi H (1980) In vitro collagen accumulation in an experimental model of pulmonary fibrosis. Exp Lung Res 1:259–270
Kilshaw PJ, Baker KC (1988) A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol Lett 18:149–154
Kilshaw PJ, Murant SJ (1990) A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol 20:2201–2207
Kilshaw PJ, Murant SJ (1991) Expression and regulation of β7 (βP) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immunol 21:2591–2597
Kim HT, Nelson EL, Clayberger C et al. (1995) γδ T cell recognition of tumor Ig peptide. J Immunol 154:1614–1623
Klein JR, Hamad M (1995) γδ T cells, antigen recognition and intestinal immunity. Immunol Today 16:108–109
Kumar RK, Watkins SG, Lykke AW (1985) Pulmonary responses to bleomycin-induced injury: an immunomorphological and electron microscopic study. Exp Pathol 28:33–43
Lanier LL, Ruitenberg, JJ, Phillips JH (1986) Human CD3+ T lymphocytes that express neither DC4 nor CD8 antigens. J Exp Med 164:339–344
Lazo JS, Humphreys CJ (1983) Lack of metabolism as the biochemical basis of bleomycin-induced pulmonary toxicity. Proc Natl Acad Sci USA 80:3064–3068
Lindenschmidt RC, Tryka AF, Godfrey GA et al. (1986) Intratracheal versus intravenous administration of bleomycin in mice: acute effects. Toxicol Appl Pharmacol 85:69–77
Lundqvist C, Baranov V, Teglund S et al. (1994) Cytokine profile and ultrastructure of intraepithelial γδ T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol 153:2302–2312
Micklem KJ, Dong Y, Willis A et al. (1991) HML-1 antigen on mucosa-associated T cells, activated cells, and hairy leukemic cells is a new integrin containing the β7 subunit. Am J Pathol 139:1297–1301
Nettelbladt O, Scheynius A, Bergh J et al. (1991) Alveolar accumulation of hyaluronan and alveolar cellular response in bleomycin-induced alveolitis. Eur Respir J 4:407–414
O'Brien RL, Happ MP, Dallas A et al. (1991) Recognition of a single hsp-60 epitope by an entire subset of γδ T lymphocytes. Immunol Rev 121:155–170
Raisfeld III (1980) Pulmonary toxicity of bleomycin analogs. Toxicol Appl Pharmacol 56:326–336
Roberts K, Kilshaw PJ (1993) The mucosal T cell integrin αM290β7 recognizes a ligand on mucosal epithelial cell lines. Eur J Immunol 23:1630–1635
Schieferdecker HL, Ullrich R, Weiss-Breckwoldt AN et al. (1990) The HML-1 antigen of intestinal lymphocytes is an activation antigen. J Immunol 144:2541–2549
Scott CS, Richards SJ, Roberts BE (1990) Patterns of membrane TcR αβ and TcR γδ chain expression by normal blood CD4+CD8−, CD4–CD8+, CD4–CD8dim+ and CD4–CD8-lymphocytes. Immunology 70:351–356
Selman M, Montano M, Montfort I et al. (1985) A new model of diffuse interstitial pulmonary fibrosis in rat. Exp Mol Pathol 43:375–387
Sikic BI, Young DM, Mimnaugh EG et al. (1978) Quantification of bleomycin pulmonary toxicity in mice by changes in lung hydroxy-proline content and morphometric histopathology. Cancer Res 38:787–792
Snider GL, Celli BR, Goldstein RH et al. (1978a) Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin. Am Rev Respir Dis 117:289–297
Snider GL, hayes JA, Korthy AL, (1978b) Chronic interstitial pulmonary fibrosis produced in hamster by endotracheal bleomycin: pathology and stereology. Am Rev Respir Dis 177:1099–1108
Thrall RS, Barton RW (1984) A comparison of lymphocyte populations in lung tissue and in bronchoalveolar lavage fluid of rats at various times during the development of bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis 129:279–283
Thrall RS, Phan SH, McCormick JR et al. (1981) The development of bleomycin-induced pulmonary fibrosis in neutrophil-depleted and complement-depleted rats. Am J Pathol 105:76–81
Thrall RS, Barton RW, D'Amato DA et al. (1982) Differential cellular analysis of bronchoalveolar lavage fluid obtained at various stages during the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis 126:488–492
Vuorio EI, Makela JK, Vuorio TK et al. (1989) Characterization of excessive collagen production during devleopment of pulmonary fibrosis induced by chronic silica inhalation in rats. Br J Exp Pathol 70:305–315
Woessner JF, Jr (1961) The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys 93:440–447
Zucker PK, Khouri NF, Rosenshein NB (1987) Bleomycin-induced pulmonary nodules: a variant of bleomycin pulmonary toxicity. Gynecol Oncol 28:284–291
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Braun, R.K., Ferrick, D.A., Sterner-Kock, A. et al. Comparison of two models of bleomycin-induced lung fibrosis in mouse on the level of leucocytes and T cell subpopulations in bronchoalveolar lavage. Comp Haematol Int 6, 141–148 (1996). https://doi.org/10.1007/BF00368457
Issue Date:
DOI: https://doi.org/10.1007/BF00368457