Abstract
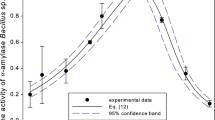
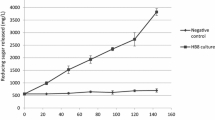
The V max of an extracellular, thermostable α-amylase from Bacillus licheniformis 44MB82 were 5.70×10-3 and 9.70×10-3 mM s-1 at 30 and 90°C, respectively, whereas the K m values were similar (0.9 mg ml-1) at both temperatures. Excluding dextrins, the dominant products from soluble starch and amylopectin hydrolysis contained less than six glucose residues. The enzyme hydrolysed amylopectin better than soluble starch. Increasing the temperature from 30 to 90°C was accompanied by an increase in the production of malto-oligosaccharides, especially maltotetrose, and this was related to the secondary hydrolysis of maltopentose and maltohexose.
Similar content being viewed by others
References
AstherM. & MeunierJ.-C. 1990 Increased thermal stability of Bacillus licheniformis α-amylase in the presence of various additives. Enzyme and Microbial Technology 12, 902–905.
BertoftE. 1989 Investigation of the fine structure of amylopectin using α- and β-amylase. Carbohydrate Research 189, 195–207.
Beschkov, M., Emanuilova, E., Kosturkova, P., Dobreva, E., Tonkova, A. & Kominkova, V. 1983 Method for obtaining of thermostable α-amylase. Bulgarian Patent 35 979.
ChangP. & SchwartzS. 1988 Characterization of the action of Bacillus subtilis α-amylase on sweet potato starch, amylose and amylopectin. Journal of Food Biochemistry 12, 191–203.
ChiangJ., AlterJ. & Sternberg-ElkhartM. 1979 Purification and characterization of a thermostable α-amylase from Bacillus licheniformis. Die Stärke 31, 88–92.
ColonnaP., BuleonA. & LemarieF. 1988 Action of Bacillus subtilis α-amylase on native wheat starch. Biotechnology and Bioengineering 31, 895–904.
DobrevaE. & IvanovaV. 1989 Characterizierung der Stärkehydrolysate nach Einwirkung einer thermostabilen α-Amylase. Acta Biotechnologica 9, 549–554.
FogartyW. 1983 Microbial amylases. In Microbial Enzymes and Biotechnology, ed FogartyW. pp. 10–40, London: Applied Science.
GovindasamyG., OatesC.G. & WongH.A. 1992 Characterization of changes of sago starch components during hydrolysis by a thermostable alpha-amylase. Carbohydrate Polymers 18, 89–100.
IvanovaV., DobrevaE. & EmanuilovaE. 1993 Purification and properties of a thermostable α-amylase from Bacillus licheniformis. Journal of Biotechnology 28, 277–289.
IvanovaV., EmanuilovaE., SedlakM. & PazlarovaJ. 1991 HPLC study of starch hydrolysis products obtained with α-amylase from Bacillus amyloliquefaciens and Bacillus licheniformis. Applied Biochemistry and Biotechnology 30, 193–201.
MadsenG., NormanB. & SlottS. 1973 A new heat stable bacterial amylase and its use in high temperature liquefaction. Die Stärke 25, 304–308.
MeddaS. & ChandraA. 1980 New strains of Bacillus licheniformis and Bacillus coagulans producing thermostable α-amylase active at alkaline pH. Journal of Applied Bacteriology 48, 47–58.
MercierC. & ColonnaP. 1988 Starch and enzymes: innovations in the products, processes and uses. Biofutur 1, 55–60.
MorganF. & PriestF. 1981 Characterization of a thermostable α-amylase from Bacillus licheniformis NCIB 6346. Journal of Applied Bacteriology 50, 107–114.
PantschevC., KlenzG. & HäfnerB. 1981 Vergleichende characterisierung von alpha-Amylaseprepäraten. Lebensmittelindustrie 28, 71–74.
ParkJ.T., YuL.P. & RollingsJ.E. 1988 Substrate effects on enzymatic depolymerization of amylose, amylopectin and glycogen. Annals of the New York Academy of Sciences 542, 53–60.
SaitoN. 1973 A thermophilic extracellular α-amylase from Bacillus licheniformis. Archives of Biochemistry and Biophysics 155, 296–298.
SomogyiM. 1952 Notes on sugar determination. Journal of Biological Chemistry 196, 19–23.
Additional information
The authors are with the Institute of Microbiology, Bulgarian Academy of Sciences, Sofia 1113. 26 Academician G. Bonchev, Bulgaria
Rights and permissions
About this article
Cite this article
Dobreva, E., Ivanova, V. & Emanuilova, E. Effect of temperature on some characteristics of the thermostable α-amylase from Bacillus licheniformis . World J Microbiol Biotechnol 10, 547–550 (1994). https://doi.org/10.1007/BF00367664
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00367664