Summary
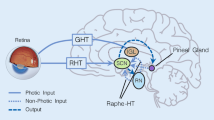
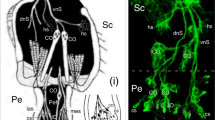
A histochemical method for demonstrating amines by fluorescence showed that the pinealocytes of the ferret contained a high concentration of a yellow fluorophore (probably 5-HT). Numerous green-fluorescent (noradrenaline-containing) nerve fibres occurred around intrapineal blood vessels, between pinealocytes and in the N. conarii (which entered the gland caudally). A collection of neuron-like cells (the pineal ganglion) lay, surrounded by a meshwork of nerve fibres, in the posterior part of the pineal. Neither the cells nor the fibres of the pineal ganglion contained monoamines, but both showed the presence of acetyl-cholinesterase which otherwise was found in the pineal only in fibres which stretched from the ganglion towards the cranial pole of the gland. The medial habenular nucleus showed a remarkable perivascular green fluorescence not seen in the lateral habenular nucleus nor anywhere else in the adjacent diencephalon and brain stem. The cells and fibres of this nucleus also contained much acetyl-cholinesterase.
Bilateral superior cervical ganglionectomy, or treating animals with reserpine, removed the green fluorescence from both pineal nerve fibres and the habenula. Ganglionectomy also resulted in a progressive alteration in the colour of the parenchymal fluorescence from yellow to green; the original yellow colour was restored by treating ganglionectomised animals with nialamide (a monoamine oxidase inhibitor). L-Dopa, 5-hydroxytryptophan or nialamide, alone or in combination, had no effect on the fluorescence of the nerve fibres or cells of the pineal, or on the habenula.
These results are related to previous findings that pinealectomy or ganglionectomy prevents the acceleration by artificial light of oestrus in ferrets.
Similar content being viewed by others
References
Abrams, M. E., Marshall, W. A., Thomson, A. P. D.: Effect of cervical sympathectomy on the onset of oestrus in ferrets. Nature (Lond.) 174, 311 (1954).
Axelrod, J., Weissbach, H.: Enzymatic O-methylation of n-acetyl serotonin to melatonin. Science 131, 1312 (1960).
—, Wurtman, R. J., Snyder, S. H.: Control of hydroxyindole O-methyl transferase activity in the rat pineal gland by environmental lighting. J. biol. Chem. 240, 949–954 (1965).
Bertler, A., Falck, B., Owman, C.: Studies on 5-hydroxytryptamine stores in pineal gland of rat. Acta physiol. scand. 68, Suppl. 249. (1964).
Bissonnette, T. H.: Modification of mammalian sex cycles: reactions of ferrets (Putorius vulgaris) of both sexes to electric light added after dark in November and December. Proc. roy. Soc. B 110, 322–336 (1932).
Bodian, D.: The staining of paraffin sections of nervous tissues with activated protargol. The role of fixatives. Anat. Rec. 69, 153–162 (1937).
Brownstein, M. J., Heller, A.: Hydroxyindole-O-methyl transferase activity: effect of sympathetic nerve stimulation. Science 162, 367–368 (1968).
Corrodi, H., Jonsson, G.: The formaldehyde fluorescence method for the histochemical demonstration of biogenic monoamines: a review of the methodology. J. Histochem. Cytochem. 15, 65–78 (1967).
Falck, B., Owman, C.: A detailed methodological description of the fluorescence method for the cellular demonstration of biogenic monoamines. Acta Univ. Lund., Sectio II, No 7 (1965).
Fiske, V. M.: Effect of light on sexual maturation, estrous cycles, and anterior pituitary of the rat. Endocrinology 29, 187–196 (1941).
Giarman, N. J., Day, M.: Presence of biogenic amines in the bovine pineal body. Biochem. Pharm. 1, 235 (1958).
Hamberger, B.: Reserpine-resistant uptake of catecholamines in isolated tissues of the rat. Acta physiol. scand. 71, Suppl. 295 (1967).
Hemmingsen, A. M., Krarup, N. B.: Rhythmic diurnal variations in the oestrus phenomena of the rat and their susceptibility to light and dark. Kgl. danske Videnstab. Selskab, Biol. Medd. 13, No 7 (1937).
Herbert, J.: Nuclear structure of the thalamus of the ferret. J. comp. Neurol. 120, 105–128 (1963).
—: The pineal gland and light-induced oestrus in ferrets. J. Endocr. 43, 625–636 (1969).
Kappers, J. A.: The development, topographical relations and innervation of the epiphysis cerebri in the albino rat. Z. Zellforsch. 2, 162–215 (1960).
—: Survey of the innervation of the epiphysis cerebri and the accessory pineal organs of vertebrates. Progr. Brain Res. 10, 87–153 (1965).
Kenny, G. C. T.: The ‘nervus conarii’' of the monkey. J. Neuropath. exp. Neurol. 20, 563–570 (1961).
Klüver, H., Barrera, E.: A method for the combined staining of cells and fibres in the nervous system. J. Neuropath. exp. Neurol. 12, 400 (1953).
Koelle, G. B., Friedenwald, J. S.: A histochemical method for localizing cholinesterase activity. Proc. Soc. exp. Biol. (N. Y.) 70, 617–622 (1949).
Kurachi, K., Iwata, R., Hirota, K.: Experimental studies on the metabolism of catecholamine in rat brain and sexual function. In: Integrative mechanism of neuroendocrine system, p. 151–163 (S. Itoh, ed). Hokkaido Medical School. 1968.
Le Gros Clark, W. E.: The nervous and vascular relations of the pineal gland. J. Anat.(Lond.) 74, 471–492 (1940).
—, McKeown, T., Zuckerman, S.: Visual pathways concerned in gonadal stimulation in ferrets. Proc. roy. Soc. B. 126, 449–468 (1939).
Lerner, A. B., Case, J. D., Takahashi, Y.: Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. biol. Chem. 235, 1992–1997 (1960).
Levin, P. M.: A nervous structure in the pineal body of the monkey. J. comp. Neurol. 68, 405–409 (1938).
Lewis, P. R., Shute, C. C. D.: The cholinergic limbic system: Projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system and the subfornical organ and supraoptic crest. Brain 90, 521–540 (1967).
Milhaud, M., Pappas, G. D.: The fibre structure of neurons and synapses of the habenula of the cat with special reference to sub-junctional bodies. Brain Res. 3, 158–173 (1966).
Mitchell, R.: Connections of the habenular and of the interpeduncular nucleus in the cat. J. comp. Neurol. 121, 441–453 (1963).
Moore, R. Y., Heller, A., Wurtman, R. J., Axelrod, J.: Visual pathways mediating pineal response to environmental light. Science 155, 220–223 (1967).
Olivier, A., Parent, A., Poirier, L. J.: Identification of the thalamic nuclei on the basis of their cholinesterase content in the monkey. J. Anat. (Lond.) 106, 37–50 (1970).
Owman, C.: Sympathetic nerves probably storing two types of monoamines in the rat pineal gland. Int. J. Neuropharmacol. 3, 105–112 (1964).
—: Localization of neuronal and parenchymal monoamines under normal and experimental conditions in the mammalian pineal gland. Progr. Brain Res. 1, 423–453 (1965).
Quay, W. B.: Circadian rhythm in rat pineal serotonin and its modifications by estrous cycle and photoperiod. Gen. comp. Endocrinol. 3, 473–479 (1963).
Reiter, R. J., Hester, R. J.: Interrelationships of the pineal gland, the superior cervical ganglia and the photoperiod in the regulation of the endocrine systems of hamsters. Endocrinology 79, 1168–1170 (1968).
Rio del Hortega, P.: Pineal gland. In: Cytology and cellular pathology of the nervous system, vol. 2, p. 635–703. (W. Penfield, ed.). New York: Hoeber 1932.
Ritzen, M.: Quantitative fluorescence microspectrophotometry of 5-hydroxytryptamine-formaldehyde products in models and mast cells. Exp. Cell Res. 45, 178–194 (1966).
Snyder, S. H., Zweig, M., Axelrod, J., Fischer, J. E.: Control of the circadian rhythm in serotonin content of the rat pineal gland. Proc. nat. Acad. Sci. (Wash.) 53, 301–305 (1965).
Strauss, W. F., Meyer, R. K.: Neural timing of ovulation in immature rats treated with gonadotrophin: effect of light. Amer. Zool. 2, 219 (1962).
Wurtman, R. J., Axelrod, J., Chu, E. W., Fischer, J. E.: Mediation of some effects of illumination on the rat estrous cycle by the sympathetic nervous system. Endocrinology 75, 266–272 (1964).
—, Kelly, D. E.: The pineal. New York: Academic Press 1968.
—, Phillips, L. S.: Melatonin synthesis in the pineal gland: control by light. Science 142, 1071–1073 (1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trueman, T., Herbert, J. Monoamines and acetyl-cholinesterase in the pineal gland and habenula of the ferret. Z. Zellforsch. 109, 83–100 (1970). https://doi.org/10.1007/BF00364933
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00364933