Abstract
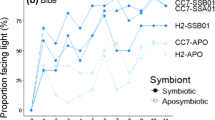
Corals within the scleractinian family Fungiidae were observed to move toward light (positive phototaxis). Negative phototactic movement was not observed in any of the specimens tested. On sandy substratum, Diaseris distorta moved faster (max. speed of 3 cm h-1) than other species. Although D. distorta has symbiotic algae, phototactic movement also was observed both in bleached corals and in those treated with a specific inhibitor (dichlorophenyl dimethyl urea) of photosynthesis. D. distorta was phototactic even on a glass plate, and climbed up a steep slope (up to 30°). Based on experiments with Fungia fungites and D. distorta, soft tissues at the peripheral region of the dise seem to be responsible for movement via peristalsis. It is suggested that positive phototaxis in symbiontbearing fungiid corals is an important trait for selection of favorable habitats.
Similar content being viewed by others
References
Abe N (1939) Migration and righting reaction of the coral, Fungia actiniformis var. palawensis Doderlein. Palao trop biol Stn Stud 28:671–694
Barnes RSK, Calow P, Olive PJW, Golding DW (1988) The invertebrates. Blackwell Scientific Publications, Oxford
Barnes DJ, Chalker BE (1990) Calcification and photosynthesis in reef-building corals and algae. In: Dubinsky Z (ed) “Coral reefs”. Ecosystems of the world 25. Elsevier, New York, pp 109–131
Battey JF, Patton JS (1984) A reevaluation of the role of glycerol in carbon translocation in zooxanthellae-coelenterate symbiosis. Mar Biol 79: 27–38
Chadwick NE (1988) Competition and locomotion in a free-living fungiid coral. J exp mar Biol Ecol 123: 189–200
Chadwick-Furman N, Loya Y (1992) Migration, habitat use, and competition among mobile corals (Scleractinia: Fungiidae) in the Gulf of Eilat, Red Sea. Mar Biol 114: 617–623
Fisk DA (1983) Free-living corals: distributions according to plant cover, sediments, hydrodynamics, depth and biological factors. Mar Biol 74: 287–294
Fredericks CA (1976) Oxygen as a limiting factor in phototaxis and in intraclonal spacing of the sea anemone Anthopleura elegantissima. Mar Biol 38: 25–28
Gill GA, Coates AG (1977) Mobility growth patterns and substrate in some fossil and recent corals. Lethaia 10: 119–134
Glynn PW (1974) Rolling stones among the scleractinia: mobile coralliths in the Gulf of Panama. In: Cameron AM et al. (ed) Proc 2nd int coral Reef Symp. Courier Mail Printing Service, Brisbane, pp 183–198
Goreau TF, Yonge CM (1968) Coral community on muddy sand. Nature, Lond 217: 421–423
Hidaka M, Shirasaka S (1992) Mechanism of phototropism in young corallites of the coral Galaxea fascicularis (L.). J exp mar Biol Ecol 157: 69–77
Hoeksema BW (1988) Mobility of free-living fungiid corals (Scleractinia), a dispersion mechanism and survival strategy in dynamic reef habitats. In: Choat JH et al. (eds) Proc 6th int coral Reef Symp. Sixth International Coral Reef Symposium Executive Committee, Townsville, pp 715–720
Hubbard JAEB (1972) Diaseris distorta an “acrobatic” coral. Nature, Lond 236: 457–459
Hubbard JAEB, Pocock YP (1972) Sediment rejection by recent scleractinian corals: a key to palaeo-environmental reconstruction. Geol Rdsch 61: 598–626
Jokiel PL, Cowdin HP (1976) Hydromechanical adaptation in the solitary free-living coral Fungia scutaria Nature, Lond 262: 212–213
Kawaguti S (1937) On the physiology of reef corals. III. Regeneration and phototropism in reef corals. Palao trop biol Stn Stud 2: 209–218
Kawaguti S (1941) On the physiology of reef corals. V. Tropisms of coral planulae, considered as a factor of distribution of the reefs. Palao trop biol Stn Stud 2: 319–328
Mackie GO (1974) Locomotion, flotation, and dispersal. In: Muscatine L, Lenhoff HM (eds) Coelenterate biology. Academic Press, New York, pp 313–357
Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky Z (ed) “Coral reefs”. Ecosystems of the world 25. Elsevier, New York, pp 75–87
Nishihira M, Poung-In S (1989) Distribution and population structure of a free-living coral, Diaseris fragilis, at Khang Khao Island in the Gulf of Thailand. Galaxea 8: 271–282
Pearse VB (1974) Modification of sea anemone behavior by symbiotic zooxanthellae: phototaxis. Biol Bull mar biol Lab, Woods Hole 147: 630–640
Sawyer SJ, Dowse HB, Shick JM (1994) Neurophysiological correlates of the behavioral response to light in the sea anemone Anthopleura elegantissima. Biol Bull mar biol Lab, Woods Hole 186: 195–201
Yamashiro H (1992) Skeletal dissolution in scleractinian corals. In: Richmond RH (ed) Proc 7th int coral Reef Symp. University of Guam, Mangilao, pp 1142–1146
Author information
Authors and Affiliations
Additional information
Communicated by T. Ikeda, Hiroshima
Rights and permissions
About this article
Cite this article
Yamashiro, H., Nishira, M. Phototaxis in Fungiidae corals (Scleractinia). Marine Biology 124, 461–465 (1995). https://doi.org/10.1007/BF00363920
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00363920