Abstract
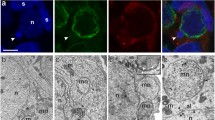
DNA contents of nuclei at different stages of gonadogenesis, gametogenesis and embryogenesis in the free-living nematode Panagrellus silusiae were measured by Feulgen microspectrophotometry. Somatic nuclei of gonadal tissue in both males and females have DNA values that range from 2C to 4C. Relative DNA values identified meiotic processes during spermatogenesis. In the adult female, developing oocytes amass an amount of nuclear DNA in excess of the expected 4C equivalent. The chromatin of these nuclei is diffuse with varyingsized clumps of Feulgen-positive material. Throughout oogenesis maturing oocytes remain uninucleolate. — The extra oocyte DNA is distributed proportionately at each of the first four cleavage divisions; thereafter, its presence is not resolvable. — A standardized microphotometric comparison indicated that the amount of DNA in the unit genome of P. silusiae is about 0.1 pg. The oviduct oocytes accumulate about 3.6 pg DNA in excess of a 4C equivalent. The kind of DNA that builds up during oogenesis in this organism is not known.
Similar content being viewed by others
References
Behme, R., Pasternak, J.: DNA base composition of some free-living nematode species. Canad. J. Genet. Cytol. 11, 993–1000 (1969)
Birnstiel, M. L., Chipchase, M., Speirs, J.: The ribosomal RNA cistrons. In: Progress in nucleic acid research and molecular biology, vol. II (J. N. Davidson and W. E. Cohn, eds.), p. 350–389. New York: Academic Press Inc. 1971
Bolla, R. I., Roberts, L. S.: Gametogenesis and chromosomal complement in Strongyloides ratti (Nematoda: Rhabdiasoidea). J. Parasit. 54, 849–855 (1968)
Brown, D. D., Blackler, A. W.: Gene amplification proceeds by a chromosome copy mechanism. J. molec Biol. 63, 75–83 (1972)
Brown, D. D., Dawid, I. B.: Specific gene amplification in oocytes. Science 160, 272–280 (1968)
Cave, M. D., Allen, E. R.: Synthesis of nucleic acids associated with a DNA-containing body in occytes of Acheta. Exp. Cell Res. 58, 201–212 (1969)
Cogging, L. W.: An ultrastructural and radioautographic study of early oogenesis in the toad Xenopus laevis. J. Cell Sci. 12, 71–93 (1973)
Crippa, M., Davidson, E. H., Mirsky, A. E.: Persistence in early amphibians of informational RNA's from the lampbrush chromosome stage of oogenesis. Proc. nat. Acad. Sci. (Wash.) 57, 885–892 (1967)
Davidson, E. H.: Gene activity in early development, p. 202–216. New York: Academic Press Inc. 1968
Floyd, A. D., Swartz, F. J.: The uterine epithelium of Ascaris lumbricoides as a model system for the study of polyploidy. Exp. Cell Res. 56, 275–280 (1969)
Foor, E. W.: Cytoplasmic bridges in the ovary of Ascaris lumbricoides. Bull. Tulane med. Fac. 27, 23–30 (1968)
Gall, J. G.: Differential synthesis of the genes for ribosomal RNA during amphibian oogenesis. Proc. nat. Acad. Sci. (Wash.) 60, 553–560 (1968)
Gall, J. G.: The genes for ribosomal RNA during oogenesis. Genetics 61, Suppl. 121–132 (1969)
Garcia, A. M., Iorio, R.: Potential sources of error in two-wavelength cytophotometry. In: Introduction to quantitative cytochemistry (G. L. Wied, ed.), p. 215–237. New York: Academic Press Inc. 1966
Jaworska, H., Lima-de-Faria, A.: Amplification of ribosomal DNA in Acheta. VI. Ultrastructure of two types of nucleolar components associated with ribosomal DNA. Hereditas (Lund) 74, 169–186 (1973)
Kato, K.: Cytochemistry and fine structure of elimination chromatin in Dytiscidae. Exp. Cell Res. 52, 507–522 (1968)
Kaulenas, M. S., Fairbairn, D.: RNA metabolism of fertilized Ascaris lumbricoides eggs during uterine development. Exp. Cell Res. 52, 233–251 (1968)
Koch, D. J., Pasternak, J., Kruuv, J.: Electronic systems for a microspectrophotometer. Med. biol. Engng 11, 239–242 (1973)
Laird, C. D.: Chromatid structure: Relationship between DNA content and nucleotide sequence diversity. Chromosoma (Berl.) 32, 378–416 (1971)
Lima-de-Faria, A.: Metabolic DNA in Tipula oleracea. Chromosoma (Berl.) 13, 47–59 (1962)
Lima-de-Faria, A.: DNA replication and gene amplification in heterochromatin. In: Handbook of molecular cytology (A. Lima-de-Faria, ed.), p. 301–325. New York: John Wiley & Sons, Inc. 1969.
Lima-de-Faria, A.: Jaworska, H., Gustafsson, T.: Release of amplified DNA from the chromomeres of Acheta. Proc. nat. Acad. Sci. (Wash.) 70, 80–83 (1973)
Macgregor, H. C.: Nucleolar DNA in oocytes of Xenopus laevis. J. Cell Sci. 3, 437–444 (1968)
Macgregor, H. C., Kezer, J.: Gene amplification in oocytes with 8 germinal vesicles from the tailed frog Ascaphus truei Stejnager. Chromosoma (Berl.) 29, 189–206 (1970)
McLaren, D. J.: The structure and development of the spermatozoan of Dipetalonema viteae (Nematoda: Filarioidea). Parasitology 66, 447–464 (1973)
Moritz, K. B.: Quantitative aspects of chromosomal composition in Ascaris megalocephala. In: Introduction to quantitative chemistry, II (G. L. Wied and G. F. Bahr, eds.), p. 57–75. New York: Academic Press Inc. 1970
Mulvey, R. H.: Oogenesis in some species of Heterodera and Meloidogyne (Nematoda: Heteroderidae). In: Nematology: Fundamentals and recent advances with emphasis on plant parasitic and soil forms (J. N. Sasser and W. R. Jenkins, eds.), p. 323–330. Chapel Hill: University of North Carolina Press 1960
Pardue, M. L., Gall, J. G.: Molecular cytogenetics. In: Molecular genetics and developmental biology (M. Sussman, ed.), p. 65–99. Englewood Cliffs, N. J.: Prentice-Hall Inc. 1972
Pasteels, J.: Recherches sur le cycle germinal chez l'Ascaris. Etude cytochimique des acides nucléiques dans l'oogénèse, la spermatogénèse et le développement chez Parascaris equorum Goetze. Arch. Biol. (Liège) 59, 405–447 (1948)
Pasternak, J., Samoiloff, M. R.: Cytoplasmic organelles present during spermatogenesis in the free-living nematode Panagrellus silusiae. Canad. J. Zool. 50, 147–151 (1972)
Patau, K.: Absorption microphotometry of irregular objects. Chromosoma (Berl.) 5, 341–362 (1952)
Perkowska, B., Macgregor, H. C., Birnstiel, M. L.: Gene amplification in the oocyte nucleus of mutant and wild type Xenopus laevis. Nature (Lond.) 217, 649–650 (1968)
Pollister, A. W., Swift, H., Rasch, E.: Microphotometry with visible light. In: Physical techniques in biological research, part C, vol. III (A. W. Pollister, ed.), p. 201–251. New York: Academic Press Inc. 1969
Rasch, E. M., Barr, H. J., Rasch, R. W.: The DNA content of sperm of Drosophila melanogaster. Chromosoma (Berl.) 33, 1–18 (1971)
Ribbert, D., Bier, K.: Multiple nucleoli and enhanced nucleolar activity in the nurse cells of the insect ovary. Chromosoma (Berl.) 27, 178–197 (1969)
Samoiloff, M. R., Pasternak, J.: Nematode morphogenesis: Fine structure of the cuticle of each stage of the nematode, Panagrellus silusiae (de Man, 1913) Goodey 1945. Canad. J. Zool. 46, 1019–1022 (1968)
Searcy, D. G., Mac Innis, A. J.: Measurements by DNA renaturation of the genetic basis of parasitic reduction. Evolution (Lawrence, Kans.) 24, 796–806 (1970)
Sin, W. C., Pasternak, J.: Number and DNA content of nuclei in the free-living nematode Panagrellus silusiae at each stage during postembryonic development. Chromosoma (Berl.) 32, 191–204 (1970)
Sulston, J. E., Brenner, S.: The DNA of Caenorhabditis elegans. Genetics 77, 95–104 (1974)
Swartz, F. J., Henry, M., Floyd, A.: Observations on nuclear differentiation in Ascaris. J. exp. Zool. 164, 297–308 (1967)
Swift, H.: The quantitative cytochemistry of RNA. In: Introduction to quantitative cytochemistry (G. L. Wied, ed.), p. 355–373. New York: Academic Press Inc. 1966
Swift, H. H., Rasch, E.: Microphotometry with visible light. In: Physical techniques in biological research (G. Oster and A. W. Pollister, eds.), p. 353–400. New York: Academic Press Inc. 1956
Tobler, H., Smith, K. D., Ursprung, H.: Molecular aspects of chromatin elimination in Ascaris lumbricoides. Develop. Biol. 27, 190–203 (1972)
Vincent, W. S., Halvorson, H. O., Chen, J. R., Shin, D.: A comparison of ribosomal gene amplification in uni- and multinucleolate oocytes. Exp. Cell Res. 57, 240–250 (1969)
Wallace, H., Morray, J., Langridge, W. H. R.: Alternative model for gene amplification. Nature (Lond.) 230, 201–203 (1971)
Westgarth-Taylor, B., Pasternak, J.: The relationship of gonadogenesis, molting and growth during postembryonic development of the free-living nematode, Panagrellus silusiae: Selective inhibition of gonadogenesis using hydroxyurea. J. exp. Zool. 183, 309–322 (1973)
Wilson, E. B.: The cell in development and heredity, p. 320–328; 1087–1093. New York: The Macmillan Co. 1928
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pasternak, J., Haight, M. DNA accumulation during oogenesis in the free-living nematode Panagrellus silusiae . Chromosoma 49, 279–298 (1975). https://doi.org/10.1007/BF00361071
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00361071