Abstract
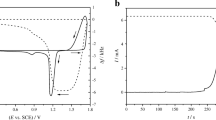
Cyclic voltammograms of a lead electrode were obtained in Na2CO3 solution as a function of the starting potential, electrolyte concentration and voltage scanning rate. The shape of the voltammograms was found to depend on the starting potential as well as the sweep number. This is probably due to changes in the activation state of the electrode surface. The first anodic portion of the voltammograms is characterized by a shoulder and two peaks corresponding to the formation of PbCO3, PbO and PbO2, respectively. The cathodic portion shows the occurrence of two peaks corresponding to the reduction of PbO2 to PbO and PbO to Pb, successively, followed by the formation of PbH2. An increase in concentration of CO 2−3 ions leads to a negative shift in the values of the peak potentials, Ep, accompanying the formation of PbO and PbO2. In addition, the current density for both the anodic oxidation peaks showed marked dependence on the concentration of the electrolyte. An increase in the scanning rate was observed to lead an increase in the size of the voltammograms. The current density of both the anodic peaks and the anodic passivation region were proportional to v1/2. Such behaviour is expected in a diffusion-controlled processes. In addition, the anodic peaks are shifted towards more positive values of potential, whereas the cathodic peaks are shifted in the negative direction, indicating irreversible formation of the passive film on the electrode surface.
Similar content being viewed by others
References
E. M. Khairy, A. A. Abdul Azim and K. M. El Sobki, J. Electroanal. Chem. 11 (1966) 282.
A. A. Abdul Azim and M. M. Anwar, Corros. Sci. 9 (1969) 245.
S. M. Abd El Haleem and A. Abd El Aal, Corros. Prevent. Cont. 29 (1982) 13.
M. Pourbaix, “Atlas of Electrochemical Equilibria” (Pergamon press, Oxford, 1966).
S. M. Abd El Haleem, E.E. Abd El Aal, A. A. Abd El Fattah and A. Abd El Aal, Res Mech. 17, (1986) 179.
R. D. Armstrong and I. Baurhoo, J. Electroanal. Chem. Interfacl. Electrochem. 34 (1972) 41.
D. D. Macdonald and D. Oven, J. Electrochem. Soc. 120 (1973) 317.
E. E. Abd El Aal, Bull. Soc. Chem. Fr., 128 (1991) 351.
S. Abd El Wanees, E. E. Abd El Aal and A. Abd El Aal Anti Corros. Meth and Mat. 28 (1991) 4.
V. I. Birss and M. T. Shevalier, J. Electrochem. Soc. 134 (1987) 802.
Idem, ibid.and M. T. Shevalier, J. Electrochem. Soc. 134 (1987) 1594.
Idem, ibid.and M. T. Shevalier, J. Electrochem. Soc. 137 (1990) 2643.
A. A. Abdul Azim and K. M. El Sobki, Corros. Sci. 12 (1972) 207.
P. Ruetschi and R. T. Angstadt, J. Electrochem. Soc. 111 (1964) 1323.
P. Jones, H. R. Thirsk and W. F. K. Wynne-Jones, Trans. Farad. Soc. 52 (1956) 1003.
R. Bates, “Electrometric pH Measurements” (John Wiley, New York, 1954) p. 41.
P. Delehay, “New Instrumental Methods in Electrochemistry” (Interscience, New York, 1954) Ch.6.
R. Adams, “Electrochemistry at Solid Electrodes” (Marcel Dekker, New York, 1969) p. 143.
W. J. Moore, “Physical Chemistry” (Longman, London, 1978) p. 443.
S. M. Abd El Haleem and B. G. Ateya, J. Electroanal. Chem. 117 (1981) 309.
J. S. Buchanan, N. P. Freestone and L. M. Peter, ibid. 182 (1985) 383.
H. Do Duc and P. Tissot, Corros. Sci. 19 (1979) 179.
S. S. Abd El Rehim, A. M. Abd El Halim and E. E. Foad, Surf. Technol. 18 (1983) 313.
V. I. Birss and W. Waudo, Can. J. Chem. 67 (1989) 1098.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abd El Aal, E.E., Abd El Wanees, S. & Abd El Aal, A. Anodic behaviour and passivation of a lead electrode in sodium carbonate solutions. JOURNAL OF MATERIALS SCIENCE 28, 2607–2614 (1993). https://doi.org/10.1007/BF00356195
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00356195