Abstract
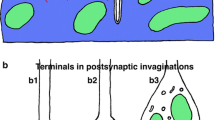
The Buccinum radula is of the rachiglossate type with two outer rows of fierce hook-like attack teeth and a medial row of straight sharp-pointed shredding teeth. Individual cells of the radular retractor muscle are 10–12 μm in diameter and separated at the closest by gaps of only 40 nm, providing areas of potential electrical contact. The cell membranes are heavily invested with long finger-like invaginations, associated with sarcoplasmic reticular cisternae, and surface caveolae; the latter are associated with the numerous dense body membrane attachment plaques found in this muscle. The radular retractor muscle possesses a significant sarcoplasmic reticulum of peripheral cisternae and deeper vesicles associated with mitochondria. The surface caveolae may result from myofilament force exerted via attachment plaques at the cell membrane, while deeper invaginations may constitute a rudimentary transverse tubular system to relay surface depolarization to associated sarcoplasmic reticular cisternae inducing calcium release to effect excitation-contraction coupling. The radular retractor muscle possesses the usual thick paramyosin and thin actin myofilaments, the latter associated with dense bodies and attachment plaques presumably to transduce force to the cell membrane. The mitochondria are unusually large and packed into dense central clusters surrounded by large deposits of glycogen granules. The nerve endings on the radular retractor muscle fibres show four different types of transmitter vesicle, presumably related to the four kinds of agonist action in this muscle, cholinergic, serotonergic, peptidergic and purinergic. All nerve endings have mixed vesicle populations, clear evidence of co-transmission. In this muscle we see a modification of usual smooth muscle structure to effect fast sustained contractions, an ultrastructural configuration functionally designed for the muscle's central role in the feeding cycle.
Similar content being viewed by others
Abbreviations
- ABRM:
-
anterior byssus retractor muscle
- EC coupling:
-
excitation-contraction coupling
- RP:
-
radular protractor muscle
- RR:
-
radular retractor muscle
- SR:
-
sarcoplasmic reticulum
- T-system:
-
transverse tubular system
References
Alohan FI, Huddart H (1981) Localization of calcium in an annelid visceral muscle by pyroantimonate deposition and by X-ray microprobe analysis. Tissue and Cell 13:525–534
Atsumi S, Sugi H (1976) Localization of calcium accumulating structures in the anterior byssus retractor muscle of Mytilus edulis and their role in the regulation of active and catch contractions. J Physiol (London) 257:549–560
Brooks DD (1982) Membrane currents associated with contraction in molluscan visceral muscle. PhD Thesis, University of Lancaster, UK
Burnstock G (1983) In: Osborn NN (ed) “Dales principle” and communication between neurons. Pergamon Press, Oxford, pp 7–35
Dorsett DA, Roberts JB (1980) A transverse tubular system at the neuromuscular junctions in a molluscan unstriated smooth muscle. Cell Tissue Res 206:251–260
Fretter V, Graham A (1962) British prosobranch molluscs 1. Ray Society, London
Hansen J, Lowy J (1957) Structure of smooth muscles. Nature (London) 180:1291–1292
Harman JW, O'Hegarty MT, Burnes CK (1962) The ultrastructure of human smooth muscle 1.Studies of cell surface and connections in normal and Achlasia esophageae smooth muscle. Exp Mol Pathol 1:204–228
Hayes RL, Kelly RE (1969) Dense bodies of the contractile system of cardiac muscle in Venus mercenaria. J Morphol 127:151–162
Hill RB, Sanger JW (1974) Anatomy and innervation of the neuromuscular junctions of the radular protractor muscle of the whelk Busycon canaliculatum. Biol Bull 147:369–385
Huddart H, Hunt S (1975) Visceral muscle—its structure and function. Blackie, Glasgow
Huddart H, Hunt S, Oates K (1977) Calcium movements during contraction in molluscan smooth muscle, and the loci of calcium binding and release. J Exp Biol 68:45–56
Hunt S (1972) The fine structure of the smooth muscle in the hypobranchial gland of the gastropod Buccinum undatum L. Tissue and Cell 4:479–492
Hunt S (1981) Molluscan visceral muscle fine structure. General structure and sarcolemmal organization in the smooth muscle of the intestinal wall of Buccinum undatum. Tissue and Cell 13:283–297
Knight GE, Hoyle CHV, Burnstock G (1992) Effects of adenine nucleosides and nucleotides on the isolated heart of the snail Helix aspersa and the slug Arion ater. Comp Biochem Physiol 101C:175–181
Nelson ID (1993) The physiology and pharmacology of muscles from the proboscis of two species of whelk, Buccinum undatum and Busycon canaliculatum. PhD Thesis, University of Lancaster, UK
Nelson ID (1994) Calcium dependency and the effect of calcium antagonists on molluscan smooth muscles from the proboscis of the whelk Buccinum undatum. J Comp Physiol B 164:147–155
Philpott DE, Kahlbrock M, Szent-Gyorgi AG (1960) Filamentous organization in molluscan smooth muscles. J Ultrastruct Res 3:254–269
Saad KHM, Huddart H (1981) Influence of noradrenaline and KCl on calcium translocation in rat vas deferens smooth muscle and its subcellular fractions. Gen Pharmacol 12:373–380
Saki T, Geffner ES, Sandow A (1970) Caffeine contracture in muscle with disrupted transverse tubules. Am J Physiol 220:712–717
Sanger JW, Hill RB (1972) Ultrastructure of the radular protractor of Busycon canaliculatum: sarcolemmic tubules and sarcoplasmic reticulum. Z Zellforsch 127:314–322
Sanger JW, Hill RB (1973) The contractile apparatus of the radular protractor muscle of Busycon canaliculatum. Proc Malacol Soc Lond 40:335–341
Sobiesczek A (1973) The fine structure of the contractile apparatus of the anterior byssus retractor muscle of Mytilus edulis. J Ultrastruct Res 43:313–343
Twarog BM, Dewey MM, Hidaka T (1973) The structure of Mytilus smooth muscle and the electrical constants of resting muscle. J Gen Physiol 61:207–211
Uehara Y, Campbell GR, Burnstock G (1976) Muscle and its innervation. Edward Arnold, London
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nelson, I.D. The relation between excitation-contraction coupling and fine structure of a molluscan muscle, the radular retractor of the whelk, Buccinum undatum . J Comp Physiol B 164, 229–237 (1994). https://doi.org/10.1007/BF00354084
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00354084