Abstract
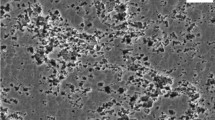
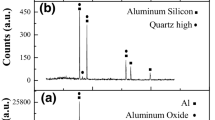
Aluminium nitride substrates were immersed in acid, basic solutions and deionized water for 1–120 h at room temperature. The corrosion rates are higher in basic solutions (NaOH and KOH) than those in acid solutions (CH3COOH, HCOOH, HNO3, HCl and H2SO4) and deionized water. The weight loss of AIN corroded in alkali aqueous reaches 70% and results in an increase in surface roughness ranging from 10 nm to 7 μm after 3 days corrosion. However, the weight loss in acid solution is only 1/700 of the alkali case. Violent chemical reactions between AIN and basic solutions were observed. Na2O, or Na2Al2O4·6H2O, is the intermediate product, and NaOH is a catalytic agent of the reaction. The surface morphology of the AIN etched by alkaline solutions is coral-like in microscopic view and appears like hills. In contrast, only several atomic layers of AIN surface are etched off in acid solutions and in deionized water. The lightly etched surface is mirror-like and flat, and the shapes of the grains are visible under the microscope, as the corrosion rate of each AIN grain varies with different crystal orientations. Consequently, after etching in acid solutions, the resulting microscopic surface morphology looks like a map of a jigsaw puzzle.
Similar content being viewed by others
References
T. Osaka, N. Hagata, E. Nakajima, I. Koiwa and K. Utsumi, J. Electrochem. Soc. 133 (1986) 2345.
K. K. Srivastava, M. Zulfequar and A. Kumar, J. Mater. Sci. 25 (1990) 2861.
K. K. Srivastava and A. Kumar, Mater. Sci. Technol. 6 (1990) 137.
R. B. Heslop and K. Jones, “Inorganic Chemistry” (Elsevier, Amsterdam, 1976) p. 341.
Y. Kurihara, T. Endoh and K. Yamada, IEEE Trans. Compos. Hybrids Manuf. Technol. 12 (1989) 330.
R. Chanchani, ibid. 11 (1988) 427.
A. Abid, R. Bensalem and B. J. Sealy, J. Mater. Sci. 21 (1986) 1301.
Y. Pauleau, A. Bouteville, J. J. Hantzpergue, J. C. Remy and A. Cachard, J. Elect. Chem. Soc. Solid State Sci. Technol. 129 (1982) 1045.
K. M. Taylor and E. Lenie, J. Electrochem. Soc. 107 (1960) 308.
G. Long and L. M. Foster, J. Am. Ceram. Soc. 42 (1959) 53.
T. Osaka, T. Asada, E. Nakajima and I. Koiwa, J. Electrochem. Soc. 135 (1988) 2578.
L. M. Sheppard, Ceram. Bull. 69 (1990) 1801.
G. A. Slack and T. F. McNelly, J. Crystal Growth 34 (1976) 263.
M. Trontelj and D. Kolar, J. Mater. Sci. 8 (1973) 136.
N. Kuramoto and Taniguchi, J. Mater. Sci. Lett. 3 (1984) 471.
B. S. Chiou and C. D. Young, in “42nd Electronic Components and Technology Conference”, San Diego, CA, Electronic Industry Association; Components, Hybrides & Manufacturing Technology Society (IEEE, New York, 1992) p. 692.
B. S. Chiou, J. H. Chang and J. G. Duh, Plat. Surf. Fin. 1 (1993) 65.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Young, C.D., Duh, J.G. Corrosion of aluminium nitride substrates in acid, alkaline solutions and water. JOURNAL OF MATERIALS SCIENCE 30, 185–195 (1995). https://doi.org/10.1007/BF00352149
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00352149