Summary
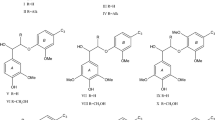
The phenyl nucleus-exchange method (NE-method) is a procedure for the degradation of lignin which allows the C−C linkages between the side chains and the phenyl nuclei to be cleaved selectively, and the phenyl nuclei to be liberated finally as polyhydric phenols. The important characteristic of this method is to take advantage of the dealkylation in diphenylmethane type structures in the presence of boron trifluoride and excess phenol, for the degradation of lignin. The lignin building units which give phenol monomers (guaiacol and/or catechol in softwood lignin) almost quantitatively by this method are noncondensed types and diphenylmethane types, and each of these units has any of the benzyl alcohol, the benzyl ether, the conjugated double bond, the α-carbonyl group and the Cα-aryl bond, in the side chain. The yields of phenyl nuclei are about 25–30% in softwood protolignins and about 8–13% in technical lignins. In this paper, the reaction theory and the degradation mechanism of lignins in the NE-method are outlined.
Similar content being viewed by others
References
Funaoka, M.; Abe, I.; Yoshimura, M. 1977: On the reaction of hydrotropic lignin with phenol and formaldehyde. Mokuzai Gakkaishi 23: 571–578
Funaoka, M.; Abe, I. 1978a: The reaction of lignin under the presence of phenol and boron trifluoride I. On the formation of catechol from MWL, dioxane lignin and kraft lignin. Mokuzai Gakkaishi 24: 256–261
Funaoka, M.; Abe, I. 1978b: The reaction of lignin under the presence of phenol and boron trifluoride II. The effect of methoxyl group and ethylenic double bond on the formation of catechol. Mokuzai Gakkaishi 24: 892–897
Funaoka, M.; Abe, I. 1980a: The reaction of lignin under the presence of phenol and boron trifluoride III. The chemical structure of lignin required for the liberation of phenyl nucleus. Mokuzai Gakkaishi 26: 334–341
Funaoka, M.; Abe, I. 1980b: The reaction of lignin under the presence of phenol and boron trifluoride IV. The structure formed by the nuclear exchange for phenol. Mokuzai Gakkaishi 26: 342–346
Funaoka, M.; Abe, I. 1982a: The reaction of lignin under the presence of phenol and boron trifluoride V. The role of boron trifluoride and phenol on the formation of catechol. Mokuzai Gakkaishi 28: 522–528
Funaoka, M.; Abe, I. 1982b: The reaction of lignin under the presence of phenol and boron trifluoride VI. The behavior of lignin building units unable to form the diphenylmethane structure. Mokuzai Gakkaishi 28: 529–534
Funaoka, M.; Abe, I. 1982c: The reaction of lignin under the presence of phenol and boron trifluoride VIII. The decomposition of softwood lignin. Mokuzai Gakkaishi 28: 627–634
Funaoka, M.; Abe, I. 1982d: The reaction of lignin under the presence of phenol and boron trifluoride IX. The decomposition of hardwood lignin. Mokuzai Gakkaishi 28: 635–643
Funaoka, M.; Abe, I. 1982e: The reaction of lignin under the presence of phenol and boron trifluoride X. The properties of residual lignin and the degradation mechanism of lignin. Mokuzai Gakkaishi 28: 705–717
Funaoka, M.; Abe, I. 1982f: The reaction of lignin under the presence of phenol and boron trifluoride XI. The decomposition of industrial lignins. Mokuzai Gakkaishi 28: 718–726
Funaoka, M. 1984: Studies on the reaction of lignin in the presence of phenol and boron trifluoride. Bull. Mie Univ. Forest No. 13: 1–154
Funaoka, M.; Abe, I. 1985: Degradation of protolignin by the nuclear exchange method. Mokuzai Gakkaishi 31: 671–676
Funaoka, M.; Abe, I. 1986: Condensation degree of softwood protolignin. Bull. Fac. Agr. Mie Univ. No. 73: in press
Goldstein, I. S. 1975: Potential for converting wood into plastics. Science 189: 847–852
Horiuchi, H. 1960: Fission and recombination of novolak resin molecules in the presence of acids. Kogyo Kagaku Zasshi 63: 1651–1654
Kratzl, K.; Oburger, M. 1980a: Kondensationsreaktionen von Lignin-Modellsubstanzen mit Phenol unter saurer Katalyse. Holzforschung 34: 11–16
Kratzl, K.; Oburger, M. 1980b: Kondensationsreaktion von Lignin-Modellsubstanzen mit Phenol unter saurer Katalyse. 2. Mitteilung. Holzforschung 34: 191–196
Oda, R.; Ogata, Y. 1941: Reaction of diphenylmethane and its derivatives with aluminum chloride. Kogyo Kagaku Zasshi 44: 961–963
Radziewanowski, C. 1984: Beiträge zur Kenntnis der Wirkungsweise des Aluminiumchlorids. Ber. 27: 3235–3238
Sakakibara, A.; Edashige, Y.; Takeyama, H. 1983: Solvolysis pulping (Part 1). Solvolysis pulping of Birch (Betula palatyphylla var. japonica) with cresol-water system, and the pulp shet strength. Japan Tappi 37: 423–429
Sano, Y.; Sakakibara, A. 1984: Delignification of woods by solvolysis with low molecular-weight phenols and water I. Cooking conditions and chemical structure of solvolysis lignins. Mokuzai Gakkaishi 30: 569–579
Schweers, W.; Pereira, H. N. 1972a: Über den Holzaufschluß mit Phenolen. I. Mitt. Tränkung verschiedener Hölzer mit Phenol. Holzforschung 26: 51–54
Schweers, W.; Rechy, M. 1972b: Möglichkeiten eines schwefelfreien Holzausschlusses. Papier 26: 585–590
Tsuge, O.; Tashiro, M. 1962: Studies on diarylalkanes (II). Reaction of diaryl-methanes and-ethanes with aluminium chloride (1). The Coal Tar 14: 513–519
Tsuge, O.; Tashiro, M. 1965: Studies of diarylalkanes (III). The cleavage of diarylmethanes under the influence of aluminum chloride. Bull. Chem. Soc. Japan 38: 184–188
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Funaoka, M., Abe, I. Phenyl nucleus-exchange method for the degradation of lignin. Wood Sci. Technol. 21, 261–279 (1987). https://doi.org/10.1007/BF00351398
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00351398