Abstract
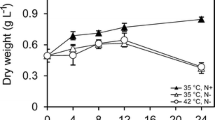
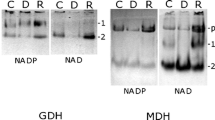
Onchidium tumidium showed a triphasic response to anoxia. Twelve hours of anoxic exposure had no effect on the glycogen content in O. tumidium. However, there were significant increases in the alanine, lactate and succinate contents in the anoxic individuals. These were accompanied by a significant decrease in the ATP content. These results suggest that O. tumidium survived the first 12 h of anoxic exposure without increasing the glycolytic flux to compensate for the lower efficiency of ATP production through anaerobic pathways. Indeed, the fructose-2,6-bisphosphate (F-2,6-P2) content and the percentage of phosphofruc-tokinase (PFK) associated with the subcellular particles remain unchanged in O. tumidium exposed to 12 h of anoxia. Hence, a reduction in the metabolic rate of these individuals might have occurred during such a period of anoxia. In contrast, in between 12 and 24 h of anoxic exposure, the glycogen content O. tumidium decreased significantly, and levelled off thereafter. A significant increase in the percentage of PFK associated with the subcellular particles was observed in individuals exposed to 24 h of anoxia. In addition, the F-2,6-P2 content of these anoxic individuals increased significantly. Taken together, these two mechanisms could activate PFK and lead to a greater glycolytic flux. Beyond 24 h of anoxic exposure, survival of O. tumidium must have required considerable suppression of metabolism as accumulation of end products and depletions of glycogen and ATP had reached constant levels.
Similar content being viewed by others
References
Bergmeyer HU, Bernt E, Schmidt E, Stork H (1974) D-Glucose. Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, Gawehn K (eds) Methods of enzymatic analysis. III. Academic Press, New York, pp 1196–1201
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Analyt Biochem 72:248–259
Brooks SPJ, de Zwaan A, Van den Thillart G, Cattani O, Cortesi P, Storey KB (1991) Differential survival of Venus gallina and Scapharca inaequivalvis during anoxic stress: covalent modification of phosphofructokinase and glycogen phosphorylase during anoxia. J comp Physiol 161(B):207–212
Brooks SPJ, Storey KB (1988) Subcellular enzyme binding in glycolytic control: in vivo studies with fish muscle. Am J Physiol 225:R289–294
Brooks SPJ, Storey KB (1990) Glycolytic enzyme binding and metabolic control in estivation and anoxia in the land snail Otala lactea. J exp Biol 151:193–204
Brooks SPJ, Storey KB (1991a) Studies in the regulation of enzyme binding during anoxia in isolated tissues of Busycon canaliculatum. J exp Biol 156:467–481
Brooks SPJ, Storey KB (1991b) Where is the glycolytic complex? A critical evaluation of the present data from muscle tissue. Fedn eur biochem Soc (FEBS) Lett 278(2):135–138
Brooks SPJ, Storey KB (1991c) A quantitative evaluation of the effect of enzyme complexes on the glycolytic rate in vivo: mathematical modelling of the glycolytic complex. J theor Biol 149:361–375
Churchill TA, Storey KB (1989) Intermediary energy metabolism during dormancy and anoxia in the land snail Otala lactea. Physiol Zool 62(5):1015–1030
Clarke FM, Shaw FD, Morton DJ (1980) Effect of electrical stimulation post mortem of bovine muscle on the binding of glycolytic enzymes. Biochem J 136:49–58
Ellington WR (1981) Energy metabolism during hypoxia in the isolated, perfused ventricle of the whelk, Busycon contrarium Condrad. J comp Physiol 142(B):457–464
Good CA, Kramer H, Somogyi M (1933) The determination of glycogen. J biol Chem 100:485–491
Gutmann I, Wahlefeld AW (1974) L-(+)-Lactate. Determination with lactate dehydrogenase and NAD. In: Bergmeyer HU, Gawehn K (eds) Methods of enzymatic analysis. III. Academic Press, New York, pp 1464–1472
Helmerhorst E, Stokes GB (1980) Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Analyt Biochem 104:130–145
Hochachka PW (1980) Anaerobic metabolism: what can and what cannot change. In: Living without oxygen: closed and open systems in hypoxia tolerance. Harvard University Press, Cambridge, Mass. pp 1–13
Hochachka PW, Somero GN (1985) Limiting oxygen availability. In: Biochemical adaptation. Princeton University Press, Princeton, New Jersey, pp 145–181
Ip YK, Chew SF, Lee CY, Wong WP, Lim ALL, Murphy DL (1993) Effects of anoxia on the activities of pyruvate kinase and phosphoenolpyruvate carboxykinase, and the production of lactate and succinate in the intertidal pulmonate Onchidium tumidium. Mar Biol 116:103–107
Job D, Cochet C, Dhien A, Chambaz EM (1978) A rapid method for screening inhibitor effects: determination of I50 and its standard deviation. Analyt Biochem 84:68–77
Kuo HJ, Malencik DA, Liou RS, Anderson SR (1986) Factors affecting the activation of rabbit muscle PFK by actin. Biochemistry (Am Chem Soc) Easton, Pa. 25:1278–1286
Lowry OH, Panssonneau JV, Hasselberger FX, Schulz DW (1964) Effect of ischaemia on known substrates and cofactors on the glycolytic pathway in brain. J biol Chem 239:18–30
Luther MA, Lee JC (1986) The role of phosphorylation in the interaction of rabbit muscle phosphofructokinase with F-actin. J biol Chem 261:1753–1759
Michaelidis B, Storey KB (1991) Evidence for phosphorylation/dephosphorylation from organs of the anoxia tolerant sea mussel Mytilus edulis. J exp Zool 257:1–9
Plaxton WC, Storey KB (1986) Glycolytic enzyme binding and metabolic control in anaerobiosis. J comp Physiol 156:635–640
Rochrig KL, Allred JB (1974) Direct enzymatic procedure for the determination of liver glycogen. Analyt Biochem 58:414–421
Storey KB (1984) Phosphofructokinase from foot muscle of the whelk Busycotypus canaliculatum: evidence for covalent modification of the enzyme during anaerobiosis. Archs Biochem Biophys 235(2):665–672
Storey KB (1985a) A re-evaluation of the Pasteur effects: new mechanisms in anaerobic metabolism. Molec Physiol 8:439–461
Storey KB (1985b) Fructose-2,6-bisphosphate and anaerobic metabolism in marine molluscs. Fedn eur biochem Soc (FEBS) Lett 182(2):245–248
Trautschold I, Lamprecht W, Schweitzer G (1985) Adenosine-5′-triphosphate. UV-method with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer J, Grassl M (eds) Methods of enzymatic analysis. VII. Academic Press, New York, pp 346–357
Van Schaftingen E (1984) D-fructose-2,6-bisphosphate. In: Bergmeyer J, Grassl M (eds) Methods of enzymatic analysis. VI. Academic Press, New York, pp 335–341
Whitwam RE, Storey KB (1991) Organ-specific regulation of phosphofructokinase during facultative anaerobiosis in the marine whelk, Busycotypus canaliculatum. Can J Zool 69:70–75
Williamson DH (1974) L-Alanine. Determination with alanine dehydrogenase. In: Bergmeyer HU, Gawehn K (eds) Methods of enzymatic analysis. IV. Academic Press, New York, pp 1679–1682
Williamson JR (1974) Succinate. In: Bergmeyer HU, Gawehn K (eds) Methods of enzymatic analysis. III. Academic Press, New York, pp 1616–1621
Author information
Authors and Affiliations
Additional information
Communicated by T. Ikeda, Hakodate
Rights and permissions
About this article
Cite this article
Lim, C.B., Low, W.P., Chew, S.F. et al. Survival of the intertidal pulmonate Onchidium tumidium during short term and long term anoxic stress. Marine Biology 125, 707–713 (1996). https://doi.org/10.1007/BF00349253
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00349253