Summary
Seasonal changes in the distribution and abundance of furanocoumarins in wild parsnip, Pastinaca sativa (Umbelliferae), were examined in a population of plants in Tompkins County, New York. Xanthotoxin, imperatorin and bergapten (linear furanocoumarins) occur in all above-ground parts of the plant; in addition, angelicin and sphondin (angular furanocoumarins) occur in umbels of some individuals. Total furanocoumarin content, as measured by percent dry weight, is greatest in reproductive parts, particularly buds and seeds; variation in concentrations between plants is greatest in vegetative structures (e.g., leaves).
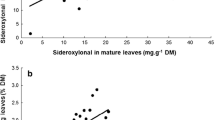
Within the plant, the distribution of furanocoumarins is significantly correlated with nitrogen, as opposed to biomass, allocation. In that nitrogen is often a factor limiting the plant growth, furanocoumarins appear to be allocated in proportion to plant tissue value; reproductive structures, obvious contributors to plant fitness, contain over ten times the amount of nitrogen and furanocoumarin contained in vegetative structures such as senescent leaves.
Stepwise multiple regression analysis revealed that generalized insect herbivores tend to feed on plants or plant parts low in furanocoumarin content and, correspondingly, low in nitrogen content. Parsnip specialists, notably Depressaria pastinacella (Lepidoptera: Oecophoridae), feed exclusively on umbels, plant parts rich in nitrogen and furanocoumarins; furanocoumarin number and content in fact account for over 60% of the variance in number of umbel feeders. These patterns conform with previous determinations of the toxicological properties of furanocoumarins. Nitrogen is known to affect growth rate, fecundity, longevity and survivorship of insect herbivores; by tolerating or detoxifying furanocoumarins, D. pastinacella can consume plant tissues containing significantly greater amounts of nitrogen than tissues consumed by generalist feeders. That the presence of D. pastinacella on individual plants is correlated with the number of furanocoumarins present is consistent with the hypothesis that parsnip specialists use angular furanocoumarins as host recognition cues.
Similar content being viewed by others
References
Alston RE (1967) Biochemical systematics. Evol Biol 1:197–305
Berenbaum M (1978) Toxicity of a furanocoumarin to armyworms: a case of biosynthetic escape from insect herbivores. Science 201:532–534
Berenbaum M (1980) Furanocoumarin chemistry, insect herbivory and coevolution in the Umbelliferae. PhD Thesis, Cornell University
Beyrich T (1966a) Xanthotoxin als Pharmakon. Pharmazie 21:282–287
Beyrich T (1966b) Die Furocumarine von Pastinaca sativa. Pharmazie 21:365–373
Boscher J (1977) Evidence of the part played by dipropyl disulphide released by leek-leaves (Allium porrum L.) in egg-laying of the leek-moth (Acrolepiopsis assectella Zell.). CRAS Ser D 284:635
Brown SA, Steck W (1973) 7-Demethylsuberosin and osthenol as intermediates in furanocoumarin biosynthesis. Phytochem 12:1315–1324
Camm EL, Wat C-K, Towers GHN (1976) An assessment of the roles of furanocoumarins in Heracleum lanatum. Can J Bot 54:2562–2566
Chapman RF (1974) The chemical inhibition of feeding by phytophagous insects. Bull Ent Res 64:339–363
Cooper-Driver GA, Swain T (1976) Cyanogenic polymorphism in bracken in relation to herbivore predation. Nature 260:604
Dabrowski ZT, Bielak B (1978) Effect of some plant chemical compounds on the behaviour and reproduction of spider mites (Acarina: Tetranychidae). Ent Exp Appl 24:117–126
Dixon AFG (1970) Quality and availability of food for a sycamore aphid population. In: Watson A (ed) Animal populations in relation to their food resources. Blackwell Scientific Publications, Oxford, p 271–287
Dolinger PM, Ehrlich PR, Fitch WL, Breedlove ED (1973) Alkaloid and predation patterns in Colorado lupine populations. Oecologia (Berl) 13:191–204
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Elliott PF (1974) Evolutionary responses of plants to seed-eaters: Pine squirrel predation on lodgepole pine. Evolution 28:221–231
Fahmy IR, Saber AH, Kadir EA (1956) Pharmacognostic study of the fruit of Pastinaca sativa cultivated in Egypt. J Pharm Pharmacol 8:653–660
Fedorin GF, Georghieyevsky VP (1975) Use of chromatospectrophotometry for the analysis of certain furanocoumarins in raw plant materials. Rast resur 11:266–268 (in Russian)
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Feeny P (1975) Biochemical coevolution between plants and their insect herbivores. In: Gilbert LE, Raven PH (eds) Coevolution of animals and plants. University of Texas Press, Austin, p 3–19
Feeny P (1976) Plant apparency and chemical defense. In: Wallace J, Mansell R (eds) Biochemical interaction between plants and insects. Rec Adv Phytochem 10:1–40
Fernald ML (1950) Gray's manual of botany (8th ed). American Book Company, New York
Flück H (1963) Intrinsic and extrinsic factors affecting the production of natural products. In: Swain T (ed) Chemical plant taxonomy. Academic Press, New York, p 167–186
Fomenko KP, Krivut BA (1972) Effects of environmental factors on the development of Ammi majus and on the accumulation of furanocoumarins in it. Rast Resur 8:217–225 (in Russian)
Fowden L, Lea PJ (1979) Mechanism of plant avoidance of autotoxicity by secondary metaboliste, especially by nonprotein amino acids. In: Rosenthal GA, Janzen DH (eds) Herbivores Their interaction with secondary metabolites. Academic Press, New York, p 135–160
Gebreyesus T (1980) Armyworm antifeedants from Clausena anisata (Willd) Hook F Ex Benth (Rutaceae). Abstr scientific working group on the use of naturally occurring plant products in pest and disease control. ICIPE, Nairobi (in press)
Griffiths GCD (1973) Studies on boreal Agromyzidae (Diptera). IV. Phytomyza miners on Angelica, Heracleum, Laserpitium, and Pastinaca (Umbellifera). Quast. Entomol. 9:219–253
Hare JD, Futuyma DJ (1978) Different effects of variation in Xanthium strumarium (Compositae) on two insect seed predators. Oecologia (Berl) 37:109–120
Hegnauer R (1973) Chemotaxonomie der Pflanzen. Bd 6. Birkhauser Verlag, Basle
Hendrix SD (1979) Compensatory reproduction in a biennial herb following insect defloration. Oecologia (Berl) 42:107–118
Hodges RW (1974) Gelechioidea Oecophoridae. The Moths of America North of Mexico Fasc 6.2. EW Classey, Ltd, London
Irving RS, Adams RP (1973) Genetic and biosynthetic relationships of monoterpenes. Rec Adv Phytochem 6:187–214
Janzen DH (1973) Community structure of secondary compounds in plants. Pure Appl Chem 34:529–538
Jermy T, Szentesi A (1978) The role of inhibitory stimuli in the choice of oviposition site by phytophagous insects. Ent Exp Appl 24:258–271
Jones DA (1966) On the polymorphism of cyanogenesis in Lotus corniculatus. I. Selection by animals. Can J Genet Cytol 8:556–567
Jones DA (1977) On the polymorphism of cyanogenesis in Lotus corniculatus L. VII. The distribution of the cyanogenic form in western Europe. Heredity 39:27–44
Knight HH (1941) The plant bugs, or Miridae, of Illinois. Bull Ill St Nat Hist Surv #22:1–234
Lichtenstein EP, Casida JE (1963) Myristicin, an insecticide and synergist occurring naturally in the edible parts of parsnip. J Agric Food Chem 11:410–415
Maksyutina NP (1965) Elucidation of composition of pastinacin and isolation of sphondin. Khim Prir Soedin 1:133–136 (in Russian)
Maksyutina NP, Kolesnikov DG (1965) Comparison of the furocoumarin content of different varieties of parsnip and the preparation of beroxan. Tr Botan Inst Akad Nauk SSr Ser 5 Rast Syr'e 12:76–78 (in Russian)
Maksyutina NP, Kolesnikov DG (1959) Investigation of the furocoumarins of cultivated parsnips. J Gen Chem USSR 29:3797–3800
McKenzie HA, Wallace HS (1954) The Kjeldahl deterination of nitrogen: a critical study of digestion conditions — temperature, catalyst, and oxidizing agent. Aust J Chem 7:55–70
McKey D (1974) Adaptive patterns in alkaloid physiology. Am Nat 108:305–320
McKey D (1979) The distribution of secondary compounds within plants. In: Rosenthal GA, Janzen DH (eds) Herbivores Their interaction with secondary plant metabolites. Academic Press, New York, p 55–133
Metcalf RL, Metcalf RA, Rhodes AM (1980) Cucurbitacins as kairomones for diabroticite beetles. Proc Natl Acad Sci USA 77:3769–3772
Mills HA, Jones JB (1979) Nutrient deficiencies and toxicities in plants: nitrogen. J Plant Nutrition 1:101–122
Moore LR (1978) Seed predation in the legume Crotalaria. II. Correlates of interplant variability in predation intensity. Oecologia (Berl) 34:203–223
Nie NH, Hull CH, Jenkins JG, Steinbrenner K, Bent DH (1975) Statistical package for the social sciences. McGraw-Hill, New York
Nielsen BE (1970) Coumarins of umbelliferous plants. Copenhagen: Royal Danish School of Pharmacy Chem Lab B
Nielsen JK, Dalgaard L, Larsen LM, Sorensen H (1979) Host plant selection of the horse-radish flea beetle Phyllotreta armoraciae (Coleoptera: Chrysomelidae): Feeding responses to glucosinolates from several crucifers. Ent Exp Appl 25:227–239
Nielsen JK, Larsen LM, Sorensen H (1979) Host plant selection of the horse-radish flea beetle Phyllotreta armoraciae (Coleoptera: Chrysomelidae): Identification of two flavonol glycosides stimulating feeding in combination with glucosinolates. Ent Exp Appl 26:40–48
Nuttall T (1818) Genera of North American plants. In: Evan J (ed) Classica Botanica Americana. Hafner Publishing Company, New York
Orlov YI, Sirenko LY (1969) Principal furocoumarins in Pastinaca sativa seeds. Rast Resur 5:445–447 (in Russian)
Rhoades DF, Cates RG (1976) A general theory of plant anti-herbivore chemistry. In: Wallace JW, Mansell RL (eds) Biochemical interaction between plants and insects. Rec Adv Phytochem 10:168–213
Rodman JE (1974) Differentiation and migration of Cakile (Cruciferae): Seed glucosinolate evidence. Syst Bot 1:137–148
Schery RW (1972) Plants for man (2nd ed). Englewood Cliffs: Prentice-Hall, Inc
Scott BR, Pathak MA, Mohn GR (1976) Molecular and genetic basis of furocoumarin reactions. Mutat Res 39:29–74
Šimsová J, Blažek Z (1967) Změny obsahu furokumarin i u Pastinaca sativa L. subsp eusativa Briq behěm vegetace. Česk Farm 16:22–28
Slansky F, Feeny P (1977) Stabilization of the rate of nitrogen accumulation by larvae of the cabbage butterfly on wild and cultivated food plants. Ecol Monog 47:209–228
Soine TO, Abu-Shady H, DiGangi FE (1956) A note on the isolation of bergapten and imperatorin from the fruits of Pastinaca sativa L. J Am Pharm Assoc 45:426–427
Sokal RR, Rohlf FJ (1969) Biometry. WH Freeman and Company, San Francisco
Soo Hoo CF, Fraenkel G (1966) The selection of food plants in a polyphagous insect, Prodenia eridania (Cramer). J Insect Physiol 12:693–709
Spath E (1937) Die natürlichen Cumarine. Chem Ber 70A:83–117
Starck V (1947) Percutaneous photosensitization due to handling of parsnips. Brit J Dermatol 59:40–41
Steck W, Bailey BK (1969) Characterization of plant coumarins by combined gas chromatography, ultraviolet absorption spectroscopy and NMR analysis. Can J Chem 47:3577–3583
Sturgeon K (1979) Monoterpene variation in ponderosa pine xylem resin related to western pine beetle predation. Evolution 33:803–814
Taylor WE, Bardner R (1968) Leaf injury and food consumption by larvae of Phaedon cochleariae (Coleoptera: Chrysomelidae) and Plutella maculipennis (Lepidoptera: Plutellidae) feeding on turnip and radish. Ent Exp Appl 11:177–184
Thompson JN (1977) Patch dynamics in the insect-Pastinaca sativa association. PhD Thesis, University of Illinois at Urbana-Champaign
Thompson JN (1978) Within-patch structure and dynamics in Pastinaca sativa and resource availability to a specialized herbivore. ecology 59:443–448
Rudloff E von (1975) Volatile leaf oil analysis in chemosystematic studies of North American conifers. Biochem Syst Ecol 2:131–167
Weaver CR, King DR (1954) Meadow spittlebug. Ohio Agric Exp Stat Res Bull 741:1–100
Yajima T, Kato N, Munakata K (1977) Isolation of insect anti-feeding principles in Orixa japonica Thunb. Agric Biol Chem 41:1263–1268
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berenbaum, M.R. Patterns of furanocoumarin production and insect herbivory in a population of wild parsnip (Pastinaca sativa L.). Oecologia 49, 236–244 (1981). https://doi.org/10.1007/BF00349195
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00349195