Abstract
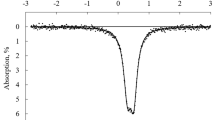
Synthetic Fe3+-melilites containing NaCaFe3+-Si2O7-, Ca2Fe3+AlSiO7- or Sr2Fe3+AlSiO7-components have been studied by 57Fe Mössbauer spectroscopy. The spectrum of åkermanite containing an NaCaFe3+Si2O7-component consists of one doublet identified to belong to Fe3+ in T1 sites. The spectra of åkermanite and gehlenite containing Ca2Fe3+ AlSiO7- or Sr2Fe3+ AlSiO7-component consist of two doublets. The inner and outer doublets are identified to belong to Fe3+ in the less distorted T1 and that in the more distorted T2 sites, respectively. The area ratios of the spectra show that the site occupancy of Fe3+ (T1) in gehlenite is less than that in åkermanite in which the distribution of Fe3+ in T1 and T2 sites is apparently random. The different distributions can be explained in terms of competition between minimizing the deficiency in the electrostatic valence and the preference of Al for T1 sites which the isomer shift measurements show to be more ionic.
Similar content being viewed by others
References
Akasaka M (1983) 57Fe Mössbauer study of clinopyroxenes in the join CaFe3+ AlSiO6-CaTiAl2O6. Phys Chem Minerals 9:205–211
Albee AL, Ray L (1970) Correction factors for electron probe microanalysis of silicates, oxides, carbonates, phosphates, and sulfates. Anal Chem 42:1408–1414
Bancroft GM, Maddock AG, Burns RG (1967) Applications of the Mössbauer effect to silicate mineralogy: I. Iron silicates of known crystal structure. Geochim Cosmochim Acta 31:2219–2246
Bartram SF (1969) Crystal structure of Y2SiBe2O7. Acta Crystallogr Sect B 25:791–795
Bence AE, Albee AL (1968) Empirical correction factors for the electron microanalysis of silicates and oxides. J Geol 76:382–403
Brown GE, Gibbs GV (1969) Oxygen coordination and the Si-O bond. Am Mineral 54:1528–1539
Edgar AD (1965) Lattice parameters of melilite solid solutions and a reconnaissance of phase relations in the system Ca2Al2SiO7(Gehlenite)-Ca2MgSi2O7(Åkermanite)-NaCaAlSi2O7 (Soda melilite) at 1000 kg/cm2 water vapor pressure. Can J Earth Sci 2:596–621
Hafner SS, Raymond M, Virgo D, Huggins FE (1976) Interpretation of the 57Fe nuclear quadrupole data for some silicate garnets. Carnegie Inst Washington Yearb 75:739–741
Heller-Kallai L, Rozenson I (1981) The use of Mössbauer spectroscopy of iron in clay mineralogy. Phys Chem Minerals 7:223–238
Huckenholz HG, Ott WD (1978) Synthesis, stability, and aluminum — iron substitution in gehlenite — ferrigehlenite solid solutions. Neues Jahrb Mineral Monatsh 12:521–536
Ito J, Peiser HS (1969) Distorted tetrahedra in strontium copper åkermanite. J Research National Bureau Standards-A. Phys Chem 73A:69–74
Kimata M (1982) Four-coordinated Co2+ cation in Ca2CoSi2O7. Naturwissensch 69:40–41
Kimata M (1983a) The crystal structure and stability of Co-åkermanite, Ca2CoSi2O7, compared with the mineralogical behaviour of Mg cation. Neues Jahrb Mineral Abh 146:221–241
Kimata M (1983b) The structure properties of synthetic Sr-åkermanite: Sr2MgSi2O7. Z Kristallogr 163:295–304
Kimata M (1984) The structural properties of synthetic Sr-gehlenite, Sr2Al2SiO7. Z Kristallogr (in press)
Kimata M, Ii N (1981) The crystal structure of synthetic åkermanite, Ca2MgSi2O7. Neues Jahrb Mineral Monatsh 15:1–10
Kimata M, Ii N (1982) The structure property of synthetic gehlenite, Ca2Al2SiO7. Neues Jahrb Mineral Abh 144:254–267
Kimata M, Ohashi H (1982) The crystal structure of synthetic gugiaite, Ca2BeSi2O7. Neues Jahrb Mineral Abh 143:202–222
Korczak P, Raaz F (1967) Verfeinerung der Kristallstruktur von Gehlenit unter Zugrundelegung des Gallium-Gehlenites. Oesterr Akad Wiss Math-Naturwiss Kl Sitzungsber 13:383–387
Louisnathan SJ (1969) Refinement of the crystal structure of hardystonite, Ca2ZnSi2O7. Z Kristallogr 130:427–437
Louisnathan SJ (1970) The crystal structure of synthetic soda melilite, CaNaAlSi2O7. Z Kristallogr 131:314–321
Louisnathan SJ (1971) Refinement of the crystal structure of a natural gehlenite, Ca2Al(Al, Si)2O7. Can Mineral 10:822–837
Ohashi H (1984) Si-O distances in X2YSi2O7 melilites and role of the electron density of the Y ions. J Jpn Assoc Mineral Petrol Econ Geol 79:235–238
Raaz F (1930) Die Struktur des synthetischen Gehlenit, 2CaO.Al2O3.SiO2. Anz Akad Wiss Wien Math-Naturwiss Kl 18:203
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172:567–570
Shinno I, Maeda Y (1981) A Fortran IV computer program LSF for analysis of Mössbauer spectra. The Reports on Earth Science College of General Education Kyushu Univ 22:13–26
Smith JV (1953) Reexamination of the crystal structure of melilite. Am Mineral 38:643–661
Warren BE (1930) The structure of melilite (Ca,Na)2(Mg,Al)1(Si,Al)2O7. Z Kristallogr 74:131–138
Warren BE, Trautz OR (1930) The structure of hardystonite Ca2ZnSi2O7. Z Kristallogr 75:525–528
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Akasaka, M., Ohashi, H. 57Fe mössbauer study of synthetic Fe3+-melilites. Phys Chem Minerals 12, 13–18 (1985). https://doi.org/10.1007/BF00348740
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00348740