Summary
Seasonal patterns of growth, 14CO2 uptake, and fluctuations in tissue titratable acidity were studied over the course of a year at a study site in the coastal plain of North Carolina.
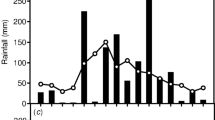
Elongation rates of Spanish moss strands were maximal in the summer and minimal in the winter. Summer maximal biomass addition rates were calculated to be 3.4 mg·month-1. Mortality of the strands was greatest in the winter months. Rates of 14CO2 uptake and fluctuations in tissue acidity were greatest in the summer over a fairly broad spectrum of environmental conditions (day and night temperatures, irradiance, length of drought). Maximal 14CO2 uptake rates (1.2 mg CO2·mg Chl-1 ·h01) were measured in May 1978. Rates of 14CO2 uptake and fluctuations in titratable acidity were inhibited below 5°C and eliminated at 0°C air temperatures.
Isothermal diurnal conditions resulted in low rates of 14CO2 uptake. Tissue water content did not appear to be a major factor controlling 14CO2 uptake rates. However, tissue wetting by rain severely reduced nighttime uptake yet stimulated low rates of daytime 14CO2 uptake. This was the only condition in which daytime 14CO2 uptake occurred, excluding the early morning and late afternoon 14CO2 uptake typical of many Crassulacean Acid Metabolism (CAM) plants.
The results suggest that tissue water content is not the major factor controlling CO2 uptake as has been found in many other CAM species; and that low temperatures limit the growth of Spanish moss in North Carolina.
Similar content being viewed by others
References
Bartholomew B (1973) Drought response in the gas exchange of Dudleya farinosa (Crassulaceae) grown under natural conditions. Photosynthetica 7:114–120
Benzing DH, Renfrow A (1971a) The significance of photosynthetic efficiency to habitat preference and phylogeny among Tillandsioid bromeliads. Bot Gaz 132:19–30
Benzing DH, Renfrow A (1971b) Significance of the patterns of CO2 exchange to the ecology and phylogeny of the Tillandsioideae (Bromeliaceae). Bull Torrey Bot Club 98:322–327
Benzing DH, Seemann J, Renfrow A (1978) The foliar epidermis in Tillandsioideae (Bromeliaceae) and its role in habitat selection. Amer J Bot 65:359–365
Bharucha FR, Joshi GV (1958) Studies in Crassulacean Metabolism in Bryophyllum calycinum under tropical conditions. J Biol Sci (India) 1:5–12
Brandon PC (1967) Temperature features of enzymes affecting Crassulacean Acid Metabolism. Plant Physiol 42:977–984
Coutinho LM (1969) Novas observações sôbre a occurrência do “Efeito de De Saussure” e suas relações com a suculência, a temperatura folhear e os movimentos estomáticos. Botânica (Brazil) 24 (331): 79–102
Eickmeier WG (1979) Eco-physiological differences between high and low elevation CAM species in Big Bend National Park, Texas. Amer Midl Nat 101:118–126
Garth RE (1964) The ecology of spanish moss (Tillandsia usneoides): Its growth and distribution. Ecology 45:470–481
Hartsock TL, Nobel PS (1976) Watering converts a CAM plant to daytime CO2 uptake. Nature (Lond) 262:574–576
Holm G (1954) Chlorophyll mutations in barley. Acta Agr Scand 4:457–471
Kluge M, Lange OL, von Eichmann M, Schmid R (1973) Diurnaler Säurerhythmus bei Tillandsia usneoides: Untersuchungen über den Weg des Kohlenstoffs sowie die Abhängigkeit des CO2-Gaswechsels von Lichtintensität, Temperatur und Wassergehalt der Pflanze. Planta (Berl) 112:357–372
Kluge M, Ting IP (1978) Crassulacean Acid Metabolism. Analysis of an Ecological Adaptation. Springer, Berlin Heidelberg New York
Lange OL, Medina E (1979) Stomata of the CAM plant Tillandsia recurvata respond directly to humidity. Oecologia (Berl) 40:357–363
Lange OL, Schulze E-D, Kappen L, Evenari M, Buschbom U (1975) CO2 exchange pattern under natural conditions of Caralluma negevensis, a CAM plant of the Negev Desert. Photosynthetica 9:318–326
Mahin DT, Lofberg RT (1970) Determination of several isotopes in tissue by wet oxidation. In: ED Bransome Jr (ed) The Current Status of Liquid Scintillation Counting. Grune & Stratton, New York pp 212–221
Martin CE (1980) Field and Laboratory Studies of Crassulacean Acid Metabolism in the Epiphyte Tillandsia usneoides L. (Spanish Moss). Unpublished PhD Dissertation, Dept of Botany, Duke University, Durham, NC, p 283
Martin CE, Siedow JN (1981) Crassulacean Acid Metabolism in the epiphyte Tillsandsia usneoides L. (Spanish moss). Responses of CO2 exchange to controlled environmental conditions. Plant Physiol 68 (in press)
McWilliams EL (1970) Comparative rates of dark CO2 uptake and acidification in the Bromeliaceae, Orchidaceae, and Euphorbiaceae. Bot Gaz 131:285–290
Medina E (1974) Dark CO2 fixation, habitat preference and evolution within the Bromeliaceae. Evolution 28:677–686
Medina E, Delgado M (1976) Photosynthesis and night CO2 fixation in Echeveria columbiana v. Poellnitz. Photosynthetica 10:155–163
Medina E, Delgado M, Troughton JH, Medina JD (1977) Physiological ecology of CO2 fixation in Bromeliaceae. Flora 166:137–152
Medina E, Troughton JH (1974) Dark CO2 fixation and the carbon isotope ratio in Bromeliaceae. Plant Sci Lett 2:357–362
Neales TF, Hew CS (1975) Two types of carbon fixation in tropical orchids. Planta (Berl) 123:303–306
Nobel PS (1976) Water relations and photosynthesis of a desert CAM plant, Agave deserti. Plant Physiol 58:576–582
Nobel PS (1977) Water relations and photosynthesis of a barrel cactus, Ferocactus acanthodes, in the Colorado Desert. Oecologia (Berl) 27:117–133
Osmond CB (1978) Crassulacean Acid Metabolism: A curiosity in context. Ann Rev Plant Physiol 29:379–414
Osmond CB, Nott DL, Firth PM (1979) Carbon assimilation patterns and growth of the introduced CAM plant Opuntia inermis in eastern Australia. Oecologia (Berl) 40:331–350
Schlesinger WH (1978) Community structure, dynamics and nutrient cycling in the Okefenokee cypress swamp-forest. Ecol Monogr 48:43–65
Schlesinger WH, Marks PL (1977) Mineral cycling and the niche of Spanish moss, Tillandsia usneoides L. Amer J Bot 64:1254–1262
Šesták Z, Čatský J, Jarvis PG (eds) (1971) Plant Photosynthetic Production. Manual of Methods. Dr W Junk NV Publ, The Hague
Smith LW (1969) Combustion and liquid scintillation determination of carbon-14 in biological samples. Anal Biochem 29:223–229
Sutton BG (1975) The path of carbon in CAM plants at night. Aust J Plant Physiol 2:377–387
Szarek SR, Ting IP (1974) Seasonal patterns of acid metabolism and gas exchange in Opuntia basilaris. Plant Physiol 54:76–81
Szarek SR, Ting IP (1975) Physiological responses to rainfall in Opuntia basilaris (Cactaceae). Amer J Bot 62:602–609
Szarek SR, Troughton JH (1976) Carbon isotope ratios in Crassulacean Acid Metabolism plants. Seasonal patterns from plants in natural stands. Plant Physiol 58:367–370
Tieszen LL, Johnson DA, Caldwell MM (1974) A portable system for the measurement of photosynthesis using 14-carbon dioxide. Photosynthetica 8:151–160
Tomlinson PB (1969) Anatomy of the Monocotyledons. III. Commelinales-Zingiberales. Clarendon Press, Oxford
Winter K, Lüttge U, Winter E, Troughton JH (1978) Seasonal shift from C3 photosynthesis to Crassulacean Acid Metabolism in Mesembryanthemum crystallinum growing in its natural environment. Oecologia (Berl) 34:225–237
Winter K, Troughton JH (1978) Carbon assimilation pathways in Mesembryanthemum nodiflorum L. under natural conditions. Z Pflanzenphysiol 88:153–162
Wong SC, Hew CS (1976) Diffusive resistance, titratable acidity, and CO2 fixation in two tropical epiphytic ferns. Amer Fern J 66:121–124
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martin, C.E., Christensen, N.L. & Strain, B.R. Seasonal patterns of growth, tissue acid fluctuations, and 14CO2 uptake in the crassulacean acid metabolism epiphyte Tjllandsia usneoides L. (Spanish moss). Oecologia 49, 322–328 (1981). https://doi.org/10.1007/BF00347592
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00347592