Summary
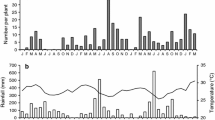
Since the successful control of prickly pear cactus by Cactoblastis cactorum in Australia, populations of plants and moths have persisted at low densities in open woodland sites. A contagious egg distribution causes overcrowding of larvae on some plants but insures low levels or no attack of other plants. This prevents extinction of plants and insects. Cactoblastis moths choose plants with characteristics which may increase the success of their larvae. Field observations and cage experiments indicate that large, green cactuses near previously attacked cactuses receive more eggs. Plants which are actively photosynthesizing are also more attractive as oviposition sites. These oviposition preferences contribute to the observed contagious egg distribution.
While open woodland Opuntia and Cactoblastis populations fluctuate around an equilibrium, pasture populations may better be described by the “hide and seek” model, with the woodland populations serving as refuges. Average plant quality and variation in quality are suggested as important components in the dynamics of this system.
Similar content being viewed by others
References
Andrewartha HG, Birch LC (1954) The Distribution and Abundance of Animals. Chicago
Barbosa P, Greenblatt J (1979) Effects of leaf age and position on larval preferences of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Can Ent 111:381–383
Birch LC (1971) The role of environmental heterogeneity in determining distribution and abundance. In: PJ den Boer and GR Gradwell (eds) Proc Adv Study Inst Dynamics Numbers Popul. p 109–128
Chew FS (1977) Coevolution of Pierid butterflies and their cruciferous foodplants. II. the distribution of eggs on potential foodplants Evolution 31:568–579
Dixon CA, Erickson JM, Kellert DN, Rothschild M (1978) Some adaptations between Danaus plexippus and its food plant, with notes on Danaus chrysippus and Euploea core. J Zool Lond 185:437–467
Dodd AP (1940) The Biological Campaign Against Prickly Pear. Commonwealth Prickly Pear Board:Brisbane
Holloway JK (1964) Projects in Biological Control of Weeds. In: P de Bach (ed) Biological Control of Insect Pests and Weeds. Chapman and Hall, London pp 650–670
Ives PM (1978) How discriminating are cabbage butterflies? Aust J Ecol 3:261–276
Jones RE, Ives PM (1979) The adaptiveness of searching and host selection behaviour in Pieris rapae (L.) Aust J Ecol 4:75–86
Krebs CJ (1972) Ecology: The Experimental Analysis of Distribution and Abundance. Harper and Row, New York
Mann J (1969) The Cactus-feeding Insects and Mites. Smithsonian Institute, Washington, D.C.. Bulletin 256
Mann J (1970) Cacti Naturalized in Australia and Their Control. Department of Lands, Brisbane
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Ann Rev Ecol Syst 11:119–161
Monro J (1967) The exploitation and conservation of resources by populations of insects. J Anim Ecol 36:531–547
Monro J (1975) Environmental variation and the efficiency of biological control—Cactoblastis in the Southern Hemisphere. In: J Kikkawa, HA Nix (eds) Managing Terrestrial Ecosystems. Proc Ecol Soc Australia 9:204–212
Myers JH (1980) Is the insect or the plant the driving force in the cinnabar moth—tansy ragwort system? Oecologia (Berl) 47:16–21
Myers JH (1976) Distribution and dispersal in populations capable of resource depletion: a simulation model. Oecologia (Berl) 23:255–269
Myers JH, Post B (1981) Plant nitrogen and insect population fluctuations: a test with the cinnabar moth-tansy ragwort system. Oecologia (Berl) 48:151–156
Osmond CB, Nott DL, Firth PM (1979) Carbon assimilation patterns and growth of the introduced CAM plant Opuntia inermis in eastern Australia. Oecologia (Berl) 40:331–350
Osmond CB, Monro J (1981) Prickly Pear. In: DJ Carr, SM Carr (eds) Plants and Man. Academic Press, Sydney, pp 194–222
Price PW (1975) Insect Ecology. John Wiley & Sons, New York
Prokopy RJ (1980) Epideictic pheromones influencing spacing patterns of phytophagous insects In: DA Nordlund, RL Jones, WJ Lewis (eds) Semiochemicals: Their Role in Pest Control. Wiley Press, New York
Rauscher MD (1979) Larval habitat suitability and oviposition preference in three related butterflies. Ecology 60:503–511
Renwick JAA, Radke CD (1980) An oviposition deterrent associated with the frass from feeding larvae of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Environ Ent 9:318–320
Ricklefs RE (1973) Ecology. Chiron Press, Portland
Rothschild M, Schoonhoven LM (1977) Assessment of egg load by Pieris brassicae (Lepidoptera, Pieridae). Nature (Lond) 266:352–355
White GG (1981) Current status of prickly pear control by Cactoblastis cactorum in Queensland. In E Del Fosse (ed) Proc V Int Symp Biol Cont Weeds, Canberra
Wicklund C (1975) The evolutionary relationship between adult oviposition preferences and larval host plant range in Papilio machaon L. Oecologia (Berl) 18:185–197
Author information
Authors and Affiliations
Additional information
This paper is dedicated to the memory of our friend Mike Sabath.
Rights and permissions
About this article
Cite this article
Myers, J.H., Monro, J. & Murray, N. Egg clumping, host plant selection and population regulation in Cactoblastis cactorum (Lepidoptera). Oecologia 51, 7–13 (1981). https://doi.org/10.1007/BF00344644
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00344644