Abstract
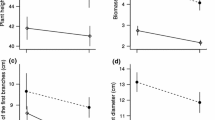
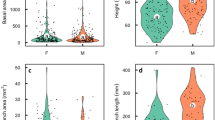
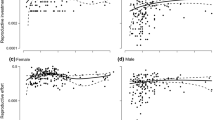
Differences in reproductive demands between the sexes of dioecious plants could cause divergence in physiology between the sexes. We found that the reproductive effort of female Silene latifolia plants increased to more than twice that of male plants or female plants that were prevented from setting fruit by lack of pollination after 4 weeks of flowering. Whole-plant source/sink ratios of pollinated females were significantly lower than those of males or unpollinated females because of investment in fruit. We hypothesized that these differences in source/sink ratio between the sexes and within females, depending on pollination, would lead to differences in leaf photosynthetic rates. Within females, we found that photosynthetic capacity was consistent with measurement of whole-plant source/sink ratio. Females that were setting fruit had 30% higher light-saturated photosynthetic rates by 28 days after flowering than females that were not setting fruit. Males, however, had consistently higher photosynthetic rates than females from 10 days after flowering onwards. Males also had approximately twice the dark respiration rates of fruiting females. We found that female reproductive structures are longer-lived and contribute more carbon to their own support than male reproductive structures. Despite the higher rates of leaf dark respiration and lower calyx photosynthetic rates, males fix more carbon than do females. We conclude that females have a sink-regulated mechanism of photosynthesis that allows them to respond to variations in fruit set. This mechanism is not, however, sufficient to explain why male S. latifolia plants have higher rates of photosynthesis, higher source/sink ratios, and lower reproductive allocation, but fail to grow larger than female plants.
Similar content being viewed by others
References
Adams WW, Winter K, Schreiber U, Schramel P (1990) Photosynthesis and chlorophyll fluorescence characteristics in relationship to changes in pigment and element composition of leaves of Platanus occidentalis L. during autumnal leaf senescence. Plant Physiol 92:1184–1190
Ågren J (1988) Sexual differences in biomass and nutrient allocation in the dioecious Rubus chamaemorus. Ecology 69:962–973
Allen GA, Antos JA (1988) Relative reproductive effort in males and females of the dioecious shrub, Oemleria cerasiformis. Oecologia 76:111–118
Arnon DI (1949) Copper enzymes in chloroplasts. Plant Physiol 24:1–15
Ashman TL, Baker I (1992) Variation in floral sex allocation with time of season and currency. Ecology 73:1237–1243
Azcón-Bieto J (1983) Inhibition of photosynthesis by carbohydrates in wheat leaves. Plant Physiol 73:681–686
Barrett JE III, Amling HJ (1978) Effects of developing fruits on production and translocation of 14C-labeled assimilates in cucumber. Hort Sci 13:545–547
Bazzaz FA, Carlson RW (1979) Photosynthetic contribution of flowers and seeds to reproductive effort of an annual colonizer. New Phytol 82:223–232
Bazzaz FA, Carlson RW, Harper JL (1979) Contribution to reproductive effort by photosynthesis of flowers and fruits. Nature 279:554–555
Boussingault JB (1868) Agronomie chimie agricole et physiologie, 2d edn. Mellet Bachelier, Paris
Carroll SB, Delph LF (in press) The effects of gender and plant architecture on allocation to flowers in dioecious Silene latifolia (Caryophyllaceae). Int J Plant Sci
Carroll SB, Mulcahy DL (1993) Progeny sex ratios in dioecious Silene latifolia (Caryophyllaceae). Am J Bot 80:551–556
Cipollini ML, Whigham DF (1994) Sexual dimorphism and cost of reproduction in the dioecious shrub Lindera benzoin (Lauraceae). Am J Bot 81:65–75
Cody ML (1966) A general theory of clutch size. Evolution 20:174–184
Conn JS (1981) Phenological differentiation between the sexes of Rumex hastalulus: niche partitioning or different optimal reproductive strategies? Bull Torrey Bot Club 108:374–378
Correns C (1928) Bestimmung, Vererbung und Verteilung des Geschlechtes bei den hoheren Pflanzen. Handb Vererbungswiss 2:1–138
Dawson TE, Bliss LC (1989) Patterns of water use and the tissue water relations in the dioecious shrub, Salix arctica: the physiological basis for habitat partitioning between the sexes. Oecologia 79:332–343
Dawson TE, Bliss LC (1993) Plants as mosaics: leaf-, ramet- and gender-level variation in the physiology of the dwarf willow, Salix arctica. Funct Ecol 7:293–304
Dawson TE, Ehleringer JR (1993) Gender-specific physiology, carbon isotope discrimination, and habitat distribution in boxelder, Acer negundo. Ecology 74:798–815
Delph LF (1990) Sex-differential resource allocation patterns in the subdioecious shrub Hebe subalpina. Ecology 71:1342–1351
Delph LF, Meagher TR (1995) Sexual dimorphism masks life history trade-offs in the dioecious plant Silene latifolia. Ecology 76:775–785
Delph LF, Lu Y, Jayne LD (1993) Patterns of resource-allocation in a dioecious Carex (Cyperaceae). Am J Bot 80:607–615
Evans AS (1991) Whole-plant responses of Brassica campestris (Cruciferae) to altered sink-source relations. Am J Bot 78:394–400
Evans LT, Rawson HM (1970) Photosynthesis and respiration by the flag leaf and components of the ear during grain development in wheat. Aust J Biol Sci 23:245–254
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Farrar JF (ed) (1993) Sink strength: what is it and how do we measure it? Plant Cell Environ 16:1013–1046
Fox JF, Stevens GC (1991) Costs of reproduction in a willow: experimental responses versus natural variation. Ecology 72:1013–1023
Fujii JA, Kennedy RA (1985) Seasonal changes in the photosynthetic rate in apple trees: A comparison between fruiting and nonfruiting trees. Plant Physiol 78:519–524
Gadgil M, Bossert WH (1970) Life historical consequences of natural selection. Am Nat 104:1–24
Galen C, Dawson TE, Stanton ML (1993) Carpels as leaves: meeting the carbon cost of reproduction in an alpine buttercup. Oecologia 95:187–193
Gehring JL (1993) Temporal patterns in the development of sexual dimorphisms in Silene latifolia (Caryophyllaceae). Bull Torrey Bot Club 120:405–416
Gehring JL, Linhart YB (1993) Sexual dimorphisms and response to low resources in the dioecious plant, Silene latifolia (Caryophyllaceae). Int J Plant Sci 154:152–162
Gehring JL, Monson RK (1994) Sexual differences in gas exchange and response to environmental stress in dioecious Silene latifolia (Caryophyllaceae). Am J Bot 81:166–174
Goldschmidt EE, Huber SC (1992) Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol 99:1443–1448
Hansen P (1970) 14C-Studies on apple trees. VI. The influence of the fruit on the photosynthesis of the leaves, and the relative photosynthetic yields of fruits and leaves. Physiol Plant 23:805–810
Herold A (1980) Regulation of photosynthesis by sink activity-the missing link. New Phytol 86:131–144
Herold A, McGee EEM, Lewis DH (1980) The effect of orthophosphate concentration and exogenously supplied sugars on the distribution of newly fixed carbon in sugar beet leaf discs. New Phytol 85:1–13
Houssard C, Thompson JD, Escarre J (1994) Do sex-related differences in response to environmental variation influence the sex-ratio in the dioecious Rumex acetosella? Oikos 70:80–90
Koch KE, Nolte KD, Duke ER, McCarty DR, Avigne WT (1992) Sugar levels modulate differential expression of maize sucrose synthase genes. Plant Cell 4:59–69
Kapp A, Stitt M (1994) Influence of high carbohydrate content on the activity of plastidic and cytosolic isoenzyme pairs in photosynthetic tissues. Plant Cell Environ 17:861–866
Krapp A, Hofmann B, Schäfer C, Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the “sink regulation” of photosynthesis? Plant J 3:817–828
Laporte MM (1994) Sex-specific physiology and source-sink relations in Silene latifolia. M.A. Thesis. Indiana University, Bloomington, Indiana, USA
Lawrence CW (1963) Genetic studies of wild populations of Melandrium. II. Flowering time and plant height. Heredity 18:149–163
Lokker C, Susko D, Lovett Doust L, Lovett Doust J (1994) Population genetic structure of Vallisneria americana, a dioecious clonal macrophyte. Am J Bot 81:1004–1012
Lovett Doust J, Lovett Doust L (1987) Leaf demography and clonal growth in female and male Rumex acetosella. Ecology 68:2056–2058
Lovett Doust J, O'Brien G, Lovett Doust L (1987) Effect of density on secondary sex characteristics and sex ratio in Silene alba (Caryophyllaceae). Am J Bot 74:40–46
Lyons EE, Miller D, Meagher TR (1994) Evolutionary dynamics of sex ratio and gender dimorphism in Silene latifolia. I. Environmental effects. J Hered 85:196–203
Marshall C, Watson MA (1992) Ecological and physiological aspects of reproductive allocation. In: Marshall C, Grace J (eds) Fruit and seed production. Cambridge University Press, Cambridge, pp 173–202
Marshall JD, Dawson TE, Ehleringer JR (1993) Gender-related differences in gas exchange are not related to host quality in the xylem-tapping mistletoe, Phoradendron juniperum (Viscaceae). Am J Bot 80:641–645
Meagher TR (1992) The quantitative genetics of sexual dimorphism in Silene latifolia (Caryophyllaceae). I. Genetic variation. Evolution 46:445–457
Mitton and Grant (1980) Observations on the ecology and evolution of quaking aspen, Populus tremuloides, in the Colorado Front Range. Am J Bot 67:202–209
Nigtevecht G van (1966) Genetic studies in dioecious Melandrium I. Sex-linked and sex influenced inheritance in M. album and M. dioicum. Genetica 37:281–306
Neales TF, Incoll LD (1968) The control of leaf photosynthesis rate by the level of assimilate concentration in the leaf: a review of the hypothesis. Bot Rev 34:107–125
Popp JW, Reinartz IA (1988) Sexual dimorphism in biomass allocation and clonal growth of Xanthoxylum americanum. Am J Bot 75:1732–1741
Primack RB, Miao SL, Becker KR (1994) Costs of reproduction in the pink lady's slipper orchid (Cypripedium acaule): Defoliation, increased fruit production, and fire. Am J Bot 81:1083–1090
Putwain PD, Harper JL (1972) Studies in the dynamics of plant populations. V. Mechanisms governing the sex ratio in Rumex acetosa and R. acetosella. J Ecol 60:113–129
Reekie EG, Bazzaz FA (1987) Reproductive effort in plants. II. Does carbon reflect the allocation of other resources? Am Nat 129:897–906
Reznick D (1983) The structure of guppy life histories: the trade-off between growth and reproduction. Ecology 64:862–873
Sage RF, Sharkey TD (1987) The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiol 84:658–664
Sakai AK, Burris TA (1985) Growth in male and female bigtooth aspen (Populus grandidentata). Ecology 68:2031–2033
Sawada S, Hasegawa Y, Kasai M, Sasaki M (1989) Photosynthetic electron transport and carbon metabolism during altered source/sink balance in single-rooted soybean leaves. Plant Cell Physiol 30:691–698
Schecter I, Proctor JTA, Elfving DC (1994) Carbon exchange rate and accumulation in limbs of fruiting and nonfruiting apple trees. J Am Soc Hort Sci 119:150–156
Sharkey TD (1985) Photosynthesis in intact leaves of C3 plants: Physics, physiology and rate limitations. Bot Rev 51:53–105
Sharkey TD, Vanderveer PJ (1989) Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol 91:679–684
Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2:1027–1038
Sonnewald U, Willmitzer L (1992) Molecular approaches to sink-source interactions. Plant Physiol 99:1267–1270
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Stitt M, Schaewen A von, Willmitzer L (1990) “Sink” regulation of photosynthetic metabolism in transgenic tobacco plants expressing yeast invertase in their cell wall involves a decrease of the Calvin-cycle enzymes and an increase of glycolytic enzymes. Planta 183:40–50
Tennessen DJ, Singsaas EL, Sharkey TD (1994) Light-emitting diodes as a light source of photosynthesis research. Photosynth Res 39:85–92
Tuomi J, Hakala T, Haukioja E (1983) Alternative concepts of reproductive effort, costs of reproduction, and sclection in life-history evolution. Am Zool 23:25–34
Walker DA, Herold A (1977) Can the chloroplast support photosynthesis unaided? Plant Cell Physiol 51:295–310
Wallace CS, Rundel PW (1979) Sexual dimorphism and resource allocation in male and female shrubs of Simmondsia chinensis. Oecologia 44:34–39
Watson MA (1984) Developmental constraints: Effect on population growth and patterns of resource allocation in a clonal plant. Am Nat 123:411–426
Watson MA, Casper BB (1984) Morphogenetic constraints on patterns of carbon distribution in plants. Annu Rev Ecol Syst 15:233–258
Willson MF (1986) On the costs of reproduction in plants: Acer negundo. Am Midland Nat 115:204–207
Ye D, Oliveira M, Veuskens J, Wu Y, Installe P, Hinnisdaels S, Truong AT, Brown S, Mouras A, Negrutiu I (1991) Sex determination in the dioecious Melandrium. The X/Y chromosome system allows complementary cloning strategies. Plant Sci 80:93–106
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Laporte, M.M., Delph, L.F. Sex-specific physiology and source-sink relations in the dioecious plant Silene latifolia . Oecologia 106, 63–72 (1996). https://doi.org/10.1007/BF00334408
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00334408