Summary
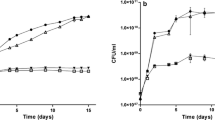
The ability of the Klebsiella pneumoniae nifL gene product to antagonise NIFA mediated transcriptional activation from the nifH promoter in vivo was inhibited either by metal deprivation, or by the presence of the iron chelators EDDA or Desferal in the growth medium. This inhibition of the repressive activity of NIFL was reversed by the addition of ferrous or manganous ions to the medium but was unaffected by other transition metals. The dependence on metal ions for NIFL activity was observed when NIFL was overexpressed and when cultures were exposed to oxygen or high levels of fixed nitrogen. Immunochemical evidence suggests that NIFL and NIFA associate to form a functional protein complex. Metal ions are apparently not required for the formation of this complex.
Similar content being viewed by others
References
Adman E, Watenpaugh KD, Jensen LH (1975) NH-S hydrogen bonds in Peptococcus aerogenes ferredoxin, Clostridium pasteurianum rubredoxin and Chromatium high potential iron protein. Proc Natl Acad Sci USA 72:4854–4858
Albright LM, Huala E, Gu Q, Ausubel FM (1988) Regulation of R. meliloti NifA function. In: Bothe H, Bruijn FJ de, Newton WE (eds) Nitrogen fixation: Hundred years after. Fischer, Stuttgart, New York, pp 345–349
Arnott M, Sidoti C, Hill S, Merrick M (1989) Deletion analysis of the nitrogen fixation regulatory gene, nifL, of Klebsiella pneumoniae. Arch Microbiol 151:180–182
Austin S, Henderson N, Dixon R (1987) Requirements for transcriptional activation in vitro of the nitrogen regulated glnA and nifLA promoters from Klebsiella pneumoniae: dependence on activator concentration. Mol Microbiol 1:92–100
Bagg A, Neilands JB (1987a) Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26:5471–5477
Bagg A, Neilands JB (1987b) Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev 51:509–518
Berg JM (1986) Potential metal-binding domains in nucleic acid binding proteins. Science 232:485–487
Brooks SJ, Collins, JJ, Brill WJ (1984) Repression of nitrogen fixation in Klebsiella pneumoniae at high temperature. J Bacteriol 157:460–464
Buchanan-Wollaston V, Cannon MC, Cannon FC (1981a) The use of cloned nif (nitrogen fixation) DNA to investigate transcriptional regulatoon of nif gene expression in Klebsiella pneumoniae. Mol Gen Genet 184:102–106
Buchanan-Wollaston V, Cannon MC, Beynon JL, Cannon FC (1981b) Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature 294:776–778
Buck M, Miller S, Drummond M, Dixon R (1986) Upstream activator sequences are present in the promoters of nitrogen fixation genes. Nature 320:374–378
Buck M, Cannon W, Woodcock J (1987a) Transcriptional activation of the Klebsiella pneumoniae nitrogenase promoter may involve DNA loop formation. Mol Microbiol 1:243–249
Buck M, Woodcock J, Cannon W, Mitchenall L, Drummond M (1987b) Positional requirements for the function of nif-specific upstream activator sequences. Mol Gen Genet 210:140–144
Cannon M, Hill S, Kavanagh E, Cannon F (1985) A molecular genetic study of nif expression in Klebsiella pneumoniae at the level of transcription, translation and nitrogenase activity. Mol. Gen Genet 198:198–206
Casadaban MJ, Martinez-Arias A, Shapira SK, Chou J (1983) β-Galactosidase gene fusions for analysing gene expression in E. coli and yeast. Methods Enzymol 100:293–308
Collins JJ, Brill WJ (1985) Control of Klebsiella pneumoniae nif mRNA synthesis. J Bacteriol 162:1186–1190
Collins JJ, Roberts GP, Brill WJ (1986) Post-transcriptional control of Klebsiella pneumoniae nif mRNA stability by the nifL product. J Bacteriol 168:173–178
Dixon R (1988) Genetic regulation of nitrogen fixation. In: Cole JA, Ferguson SJ (eds) The Nitrogen and Sulphur Cycles. Cambridge University Press, Cambridge, pp 417–438
Dixon R, Kennedy C, Kondorosi A, Krishnapillai V, Merrick M (1977) Complementation analysis of Klebsiella pneumoniae mutants defective in nitrogen fixation. Mol Gen Genet 157:189–198
Drummond M, Wootton J (1987) Sequence of nifL from Klebsiella pneumoniae: mode of action and relationship to two families of regulatory proteins. Mol Microbiol 1:37–44
Eady R, Robson R (1984) Characteristics of N2 fixation in Molimited batch and continuous cultures of Azotobacter vinelandii. Biochem J 224:853–862
Filser M, Merrick M, Cannon F (1983) Cloning and characterisation of nifLA regulatory mutations from Klebsiella pneumoniae. Mol Gen Genet 191:485–491
Fischer H-M, Hennecke H (1987) Direct response of Bradyrhizobium japonicum nifA-mediated nif gene regulation of cellular oxygen status. Mol Gen Genet 209:621–626
Fischer H-M, Bruderer T, Hennecke H (1988) Essential and nonessential domains int he Bradyrhizobium japonicum NifA protein: identification of indispensable cysteine residues potentially involved in redox reactivity and/or metal binding. Nucleic Acids Res 16:2207–2224
Gussin GN, Ronson CW, Ausubel FM (1986) Regulation of nitrogen fixation genes. Annu Rev Genet 20:567–591
Hassan HM, Moody CS (1987) Regulation of manganese-containing superoxide dismutase in Escherichia coli. J Biol Chem 262:17173–17177
Hawkins FKL, Johnston AWB (1988) Transcription of a Rhizobium leguminosarum biovar phaseoli gene needed for melanin synthesis is activated by nifA of Rhizobium and Klebsiella pneumoniae. Mol Microbiol 2:331–337
Hess JF, Oosawa K, Kaplan N, Simon MI (1988) Phosphorylation of three proteins in the signalling pathway of bacterial chemotaxis. Cell 53:79–87
Hill S, Kennedy C, Kavanagh E, Goldberg RB, Hanau R (1981) Nitrogen fixation gene (nifL) involved in oxygen regulation of nitrogenase synthesis in K. pneumoniae. Nature 290:424–426
Hirschman J, Wong P-K, Sei K, Keener J, Kustu S (1985) Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro. Evidence that the ntrA product is a σ factor. Proc Natl Acad Sci USA 82:7525–7529
Holmgren A (1986) Thioredoxin and glutaredoxin systems: An overview. In: Holmgren A, Bränden C-I, Jörnvall H, Sjöberg B-M (eds) Thioredoxin and glutaredoxin systems: Structure and function. Raven Press, New York, pp 1–9
Hunt T, Magasanik B (1985) Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG and glnL. Proc Natl Acad Sci USA 82:8453–8457
Keener J, Kustu S (1988) Protein kinase and phosphorylation phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci USA 85:4976–4980
Kennedy C (1977) Linkage map of the nitrogen fixation (nif) genes in Klebsiella pneumoniae. Mol Gen Genet 157:199–204
Kleiner D, Paul W, Merrick MJ (1988) Construction of multicopy expression vectors for regulated overproduction of proteins in Klebsiella pneumoniae and other enteric bacteria. J Gen Microbiol 134:1779–1784
Ludden PW, Roberts GP, Lowery RG, Fitzmaurice WP, Saari LL, Lehman L, Lies D, Woehle D, Wirt H, Murrell SA, Pope MR, Kanemoto RH (1988) Regulation of nitrogenase activity by reversible ADP-ribosylation of dinitrogenase reductase. In: Bothe H, Bruijn FM de, Newton WE (eds) Nitrogen fixation: Hundred years after. Fischer, Stuttgart, New York, pp 157–162
Lorezo V de, Wee S, Henero M, Neilands JB (1987) Operator sequences of the aerobacterin operon of plasmid CoIV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol 169:2624–2630
MacNeil D (1981) General method using Mu-Mud1 dilysogens to determine the direction of transcription of and generate deletions in the glnA region of Escherichia coli. J Bacteriol 146:260–268
Merrick M, Hill S, Hennecke H, Hahn M, Dixon R, Kennedy C (1982) Repressor properties of the nifL gene product in Klebsiella pneumoniae. Mol Gen Genet 185:75–81
Minchin SD, Austin S, Dixon RA (1988) The role of activator binding sites in transcriptional control of the divergently transcribed nifF and nifLA promoters from Klebsiella pneumoniae. Mol Microbiol 2:433–442
Morett E, Buck M (1988) NifA-dependent in vivo protection demonstrates that the upstream activator sequence of nif promoters is a protein binding site. Proc Natl Acad Sci USA, in press
Neilands JB (1981) Microbial iron compounds. Annu Rev Biochem 50:715–731
Ninfa AJ, Magasanik B (1986) Covalent modification of the glnG product NRI by the glnL product NRII regulates transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci USA 83:5909–5913
Nixon TB, Ronson CW, Ausubel FM (1986) Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci USA 83:7850–7854
Nordlund S, Noren A (1984) Dependence on divalent cations of the activation of inactive Fe protein of nitrogenase from Rhodospirillum rubrum. Biochim Biophys Acta 791:21–27
Putnam SL, Koch AL (1975) Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of β-galactosidase. Anal Biochem 63:350–360
Ronson CW, Nixon TB, Ausubel FM (1987) Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell 49:579–581
Schäffer S, Hantke K, Braun V (1985) Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet 200:110–113
Smith A, Hill S, Anthony C (1988) A haemoprotein is not involved in the control by oxygen of enteric nitrogenase synthesis. J Gen Microbiol 134:1499–1507
Spiro S, Guest JR (1988) Inactivation of the FNR protein of Escherichia coli by targeted mutagenesis in the N-terminal region. Mol Microbiol 2:701–707
Tuli R, Merrick MJ (1988) Over-production and characterisation of the nifA gene product of Klebsiella pneumoniae — the transcriptional activator of nif gene expression. J Gen Microbiol 134:425–432
Unden G, Guest JR (1985) Isolation and characterisation of the Fnr protein, the transcriptional regulator of electron transport in Escherichia coli. Eur J Biochem 146:193–199
Williams RJP (1982) Free manganese(II) and iron(II) cations can act as intracellular controls. FEBS Lett 140:3–10
Yoch DC (1979) Manganese, an essential trace element for nitrogen fixation by Rhodospirillum rubrum and Rhodopseudomonas capsulata: role in nitrogenase regulation. J Bacteriol 140:987–995
Author information
Authors and Affiliations
Additional information
Communicated by H. Hennecke
Rights and permissions
About this article
Cite this article
Henderson, N., Austin, S. & Dixon, R.A. Role of metal ions in negative regulation of nitrogen fixation by the nifL gene product from Klebsiella pneumoniae . Mol Gen Genet 216, 484–491 (1989). https://doi.org/10.1007/BF00334394
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00334394