Abstract
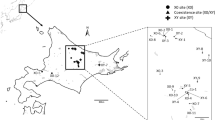
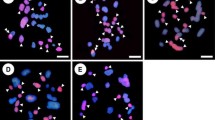
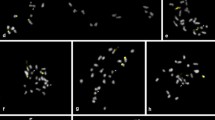
Discovery of permanent hybridity in the very large chromosomes of Gibasis pulchella (Commelinaceae) has allowed specific identification of segmental interchanges in complex heterozygotes. The interchanges are confined to terminal regions, are sometimes very small, and may be unequal in size. Breakpoints have occurred close to major C-bands, probably at euchromatin/heterochromatin boundaries. Complete and disjunctional ring formation at meiosis results in the segregation of two Renner complexes, each of which can be specifically identified with C-banding. The complex carrying the interchanges is usually transmitted through the pollen. Certain chromosomes that have undergone more extensive change than the rest of the complement may have some special significance. There is evidence of small duplications within heterozygous genomes. Permanent hybridity in different organisms may have quite different origins, possibly initiated by major karyotype repatterning following the activation of transposons that generate chromosome breakage.
Similar content being viewed by others
Abbreviations
- p :
-
Short arm
- q :
-
long arm
- M :
-
metacentric
- SM :
-
submetacentric
- BS :
-
bisatellited chromosome in population 4
- UB :
-
unbanded chromosome in population 4
- II :
-
bivalent; ⊙ closed meiotic ring configuration, e.g., ⊙ 10, ring of ten
- ch :
-
open chain configuration
- NOR :
-
nucleolus organising region
References
Ar-Rushdi AH (1963) The cytology of achiasmatic meiosis in the female Tigriopus. Chromosoma 13:526–536
Bhaduri PN (1943) Cytological analysis of structural hybridity in Rhoeo discolor Hance. J Genet 44:73–85
Carr RL, Carr GD (1983) Chromosome races and structural heterozygosity in Calycadenia ciliosa Greene (Asteraceae). Am J Bot 70:744–755
Catcheside DG (1932) The chromosomes of a new haploid Oenothera. Cytologia 4:68–113
Catcheside DG (1947) A duplication and deficiency in Oenothera. J Genet 48:99–110
Chinnappa CC, Victor R (1979) Achiasmatic meiosis and complex heterozygosity in female cyclopoid copepods (Copepoda, Crustacea) Chromosoma 71:227–236
Cleland RE (1972) Oenothera: cytogenetics and evolution. Academic Press, New York
Coen E, Strachan T, Brown S, Dover D (1983) On the limited independence of chromosome evolution. In:Brandham PE, Bennett MD (eds) Proc Kew Chromosome Conference II. George Allen and Unwin, London, pp 295–303
Darlington CD (1929) Ring-formation in Oenothera and other genera. J Genet 20:345–363
Darlington CD, La Cour LF (1950) Hybridity selection in Campanula. Heredity 4:217–248
Ernst H (1940) Zytogenetische Untersuchungen an haploiden Pflanzen von Antirrhinum majus. 1. Die Meiosis. Z Bot 35:161–190
Fincham JRS (1987) Patterns of flower pigmentation. Nature 325:390–39
Flavell RB, O'Dell M, Hutchinson J (1981) Nucleotide sequence organization in plant chromosomes and evidence for sequence translocation during evolution. Cold Spring Harbor Symp Quant Biol 45:501–508
Freeling M (1984) Plant transposable elements and insertion sequences. Annu Rev Plant Physiol 35:277–298
Gerasimova TI, Mizvokhi LJ, Georgier GP (1984) Transposition bursts in genetically unstable Drosophila melanogaster. Nature 309:714–716
Goldblatt P (1980) Uneven diploid chromosome numbers and complex heterozygosity in Homeria (Iridaceae). Syst Bot 5:337–340
Hizume M, Sato S, Tanaka A (1980) A highly reproducible method of nucleolus organizing regions staining in plants. Stain Technol 55:87–90
Holsinger KE, Ellstrand NC (1984) The evolution and ecology of permanent translocation heterozygotes. Am Nat 124:48–71
Jackson RC (1985) Genomic differentiation and its effect on gene flow. Syst Bot 10:391–404
James SH (1970) Complex hybridity in Isotoma petraea. II. Components of a possible evolutionary mechanism. Heredity 25:53–77
Jones K (1978) Aspects of chromosome evolution in plants. Adv Bot Res 6:120–194
Jones K, Kenton A (1984) Mechanisms of chromosome change in the evolution of the tribe Tradescantieae (Commelinaceae). In: Sharma A, Sharma AK (eds) Chromosomes in evolution of eukaryotic groups. CRC Press, Florida, pp 143–168
Jones K, Papes D, Hunt DR (1975) Contributions to the cytotaxonomy of the Commelinaceae. II. Further observations on Gibasis geniculata and its allies. Bot J Linn Soc 71:145–166
Karp A, Bright SWJ (1985) On the causes and origins of somaclonal variation. Oxford Surv Plant Mol Cell Biol 2:199–234
Kenton A (1981) Chromosome evolution in the Gibasis linearis alliance (Commelinaceae). I. The Robertsonian differentiation of G. venustula and G. speciosa. Chromosoma 84:291–304
Levan A (1942) Studies on the meiotic mechanism of haploid rye. Hereditas 28:177–211
Lewis H, Szweykowski J (1964) The genus Gayophytum (Onagraceae). Brittonia 16:343–392
Lewis KR, John B (1963) Chromosome marker. J&A Churchill Ltd, London, p 62
Lin YJ, Paddock EF (1973) Ring-position and frequency of adjacent distribution of meiotic chromosomes in Rhoeo spathacea. Am J Bot 60:685–690
McClintock B (1978) Mechanisms that rapidly reorganize the genome. Stadler Symp Univ Missouri, Columbia 10:25–47
McGuire PE (1986) Suppression of meiotic ring univalents in hybrids of Triticum aestivum L. by chromosome 7Bp. J Hered 77:222–224
Naronha-Wagner M, Mello-Sampayo T (1971) Haploids nulli 5B of Triticum aestivum. Agronomia Lusitania 33:315–322
Owens SJ (1979) The use of empty hard gelatin capsules in controlled pollinations. Euphytica 28:609–610
Owens SJ (1981) Self-incompatibility in the Commelinaceae. Ann Bot London 47:567–581
Raven PH, Gregory DP (1972) Observations of meiotic chromosomes in Gaura (Onagraceae). Brittonia 24:71–86
Schubert I, Wobus U (1985) In situ hybridization confirms jumping nucleolus organizing regions in Allium. Chromosoma 92:143–148
Schwarzacher T, Ambros P, Schweizer D (1980) Application of Giemsa banding to orchid karyotype analysis. Plant Syst Evol 134:293–297
Stack SM, Soulliere DL (1984) The relationship between synapsis and chiasma formation in Rhoeo spathacea. Chromosoma 90:72–83
Tilquin JP (1981) An unusual case of a complex heterozygote presenting no taxonomical problem in Chelidonium majus L. (Papaveraceae). Experientia 37:341–342
Walbot V, Cullis CA (1985) Rapid genomic change in higher plants. Annu Rev Plant Physiol 36:367–396
Zantovska-Stirton JM, Harborne JB (1980) Two distinctive anthocyanin patterns in the Commelinaceae. Biochem Syst Ecol 8:285–287
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kenton, A., Davies, A. & Jones, K. Identification of Renner complexes and duplications in permanent hybrids of Gibasis pulchella (Commelinaceae). Chromosoma 95, 424–434 (1987). https://doi.org/10.1007/BF00333994
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00333994