Summary
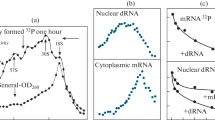
A developmentally regulated DNA sequence was isolated from a Dictyostelium discoideum genomic library by cross hybridization with a cDNA clone of cysteine proteinase I. The genomic fragment represents a different but related gene, designated cysteine proteinase II. This sequence is unique in the genome and it hybridizes to a polyA+ RNA of 1.5 kb length, which is produced after the aggregation period.
From the determination of polarity of transcription and from the nucleotide sequence, it was shown that the isolated fragment representing about a third of the gene includes its 3′ end. The open reading frame ends with a termination codon TAA and is not interrupted by intervening sequences. The flanking untranslated sequence beyond the 3′ terminus is very A+T rich (91%) and includes a sequence, ATTAAA, known to be important in polyA addition.
A striking homology in the corresponding amino acid sequence was found not only with cysteine proteinase I of Dictyostelium discoideum but also with other known cysteine proteases from plants and from mammals. The strongest homology is observed especially around the cysteine at the active site identified in some of these enzymes. Interestingly the organization of cysteine proteinase I and II of Dictyostelium discoideum differs considerably. The C terminal region of the latter corresponds to the N terminal one of the former.
Similar content being viewed by others
References
Alton TH, Lodish HF (1977) Translational control of protein synthesis during the early stages of differentiation of the slime mold Dictyostelium discoideum. Cell 12:301–310
Blumberg DD, Lodish HF (1980) Changes in the messenger RNA population during differentiation of Dictyostelium discoideum. Dev Biol 78:285–300
Bogdanovsky-Sequeval D, Ayala J, Mathieu M, Presse F, Felenbok B (1984) Characterization and regulation of the expression of a cloned sequence derived from a post aggregation regulated mRNA of Dictyostelium discoideum. Biol Cell 50:217–222
Burns DH, Beacham I (1983) A method for the ligation of DNA following isolation from low melting temperature agarose. Anal Biochem 135:48–51
Carne A, Moore CH (1978) The aming acid sequence of the tryptic peptides from actinidin a proteolytic enzyme from the fruit of actinidia chinensis. Biochem J 173:74–83
Casadaban MJ, Cohen SN (1980) Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol 138:179–207
Chandler FM, Rimkus D, Davidson N (1979) Gel electrophoretic fractionation of RNAs by partial denaturation with methylmercuric hydroxide. Anal Biochem 99:200–206
Christophe D, Brocas H, Vassart O (1982) Improved synthesis of DMM paper. Anal Biochem 120:259–261
Cockburn AF, Newkirk MJ, Firtel RA (1976) Organization of the ribosomal genes of Dictyostelium discoideum: Mapping of the non transcribed spacer regions. Cell 9:605–613
Dayhoff MO, Schwartz RM, Orcutt BC (1979) Atlas of protein. In: Dayhoff MO (ed) Sequence of structure, vol 5, Suppl. 3. pp 345–352
Felenbok B, Monier F, Guespin J (1974) Effect of the thymidine analog 5-bromo-2-deoxy uridine on Dictyostelium discoideum. Cell Diff 3:55–62
Firtel RA, Bonner JT (1972) Characterization of the genome of the cellular slime mold Dictyostelium discoideum. J Mol Biol 66:339–361
Fong D, Bonner JT (1979) Proteases in cellular slime mold development: evidences for their involvement. Proc Natl Acad Sci USA 76:6481–6485
Gal A, Nahon JL, Sala-Trepat JM (1983) Detection of rare RNA species in a complex RNA population by blot hybridization techniques: a comparative survey. Anal Biochem 132:190–194
Gerlinger P, Krust A, Le Meur M, Perrin F, Cochet M, Gannon F, Dupret D, Chambon P (1982) Multiple initiation and polyadenylated sites for the chicken ovomucoid transcription unit. J Mol Biol 162:345–364
Grunstein M, Hogness DS (1975) Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA 72:3961–3965
Jacquet M, Part D, Felenbok B (1981) Changes in the polyadenylated messenger RNA population during development of Dictyostelium discoideum. Dev Biol 81:155–166
Jacquet M, Kalekine M, Boy-Marcotte E (1985) Sequence analysis of Dictyostelium discoideum genes coding for an active DHOase in yeast. Biochimie 67:583–588
Julien J, Bogdanovsky-Sequeval D, Jacquet M, Felenbok B (1984) Expression of a spore specific gene in Dictyostelium discoideum. Cell Differ 15:37–42
Juna A, Sippel AE, Greg M, Shutz G (1980) Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci USA 77:5759–5763
Kafatos FC, Jones CW, Efstratiadis A (1979) Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acid Res 7:1541
Kimmel AR, Firtel RA (1980) Intervening sequences in a Dictyostelium gene that encodes a low abundance class mRNA. Nucleic Acid Res 8:5599–5610
Kimmel AR, Firtel RA (1983) Sequence organization in Dictyostelium: unique structure at the 5′ ends of protein coding genes. Nucleic Acid Res 11:541–552
Landfear SM, Lefebvre PA, Chung S, Lodish HF (1982) Transcriptional control of gene expression during development of Dictyostelium discoideum. Mol Cell Biol 2:1417
Loomis WF (1982) The development of Dictyostelium discoideum. Academic Press, New York
Mandel M, Higa A (1970) Calcium-dependent bacteriophage DNA infection. J Mol Biol 53:159–162
Maniatis R, Fritsch EF, Sambrook J (1982) Molecular cloning, Cold Spring Harbor Laboratory, New York
Mc Keown M, Firtel RA (1981) Evidence for sub-families of actin genes in Dictyostelium as determined by comparisons of 3′ sequences. J Mol Biol 151:593–606
Messing J (1983) New M13 vectors for cloning. Methods Enzymol 101:20–79
Mitchel REJ, Chaiken IM, Smith EL (1970) The complete amino acid sequence of papain. Distance and correction. J Biol Chem 245:3485–3492
Nichols BP, Miozzari GF, Cleemput MV, Bennett GN, Yanofsky C (1980) Nucleotide sequences of the trp G regions of Escherichia coli, Shigella dysenteriae, Salmonella thyphimurium and Serratia marcescens. J Mol Biol 142:503–517
North MJ (1982) Comparative Biochemistry of the proteinases of eucaryotic microorganisms. Microbiol Rev 46:308–340
Pabo CO, Sauer RT (1984) Protein-DNA recognition. Annu Rev Biochem 53:293–321
Rigby PWJ, Dieckmann M, Rhodes C, Berg P (1977) Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol 113:237–251
Rowekamp W, Poole S, Firtel RA (1980) Analysis of the multigene family coding the developmentally regulated carbohydrate binding protein discoidin I in D. discoideum. Cell 20:495–505
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Scrive-Menahem M, Ayala J, Jacquet M, Felenbok B (1984) Changes in protein synthesis and in RNA polyA+ population after treatment of Dictyostelium amoebae by 5-bromo-2′-deoxyuridine. Biol Cell 52:231–242
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Staden R (1982) An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acid Res 10:2051–2961
Staden R (1984) Graphic methods to determine the function of nucleic acid sequences. Nucleic Acid Res 12:521–538
Takio K, Towatari T, Katunuma N, Teller DC, Titani K (1983) Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci USA 80:3666–3670
Thomas P (1980) Hybridization of denaturated RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA 77:5201–5205
Williams JG, Lloyd MM (1979) Changes in the abundance of polyadenylated RNA during slime mould development measured using cloned molecular hybridization probes. J Mol Biol 129:19–35
Williams JG, North MJ, Mahbubani H (1985) A developmentally regulated cysteine proteinase in Dictyostelium discoideum. EMBO 4:999–1006
Author information
Authors and Affiliations
Additional information
Communicated by W. Gajewski
Rights and permissions
About this article
Cite this article
Presse, F., Bogdanovsky-Sequeval, D., Mathieu, M. et al. Structural analysis of a developmentally regulated sequence encoding for a cysteine proteinase in Dictyostelium discoideum . Molec Gen Genet 203, 324–332 (1986). https://doi.org/10.1007/BF00333975
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00333975