Summary
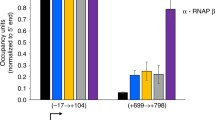
The rate of synthesis and intracellular content of the NusA protein, a transcription termination factor, were determined for wild-type and nusA and/or nusB mutants of Escherichia coli. Both the rate and content of NusA in wild-type strains were similar to that of the RNA polymerase σ subunit, a transcription initiation factor, on a molar basis, and about 30%–40% the levels of RNA polymerase ββ′ subunits. At the stationary phase of cell growth, the values increased in parallel for both transcription factors up to approximately the level of the ββ′ subunits. In nus mutants, the rate of synthesis and the content of the σ subunit were significantly increased. These observations together suggest that the two transcription factors are coordinately regulated.
Similar content being viewed by others
References
Clarke L, Carbon J (1976) A colony bank containing synthetic Col E1 hybrid plasmids representative of the entire Escherichia coli genome. Cell 9:91–99
Clewell DB (1972) Nature of Col E1 plasmid replication in Escherichia coli in the presence of chloramphenicol. J Bacteriol 110:667–670
Enami M, Ishihama A (1982) Biosynthesis of RNA polymerase in Escherichia coli, XII. Noncoordinate synthesis of core enzyme subunits after supression of cell growth. Mol Gen Genet 185:373–378
Friedman DI (1971) A bacterial mutant affecting lambda development. In: Hershey AD (eds) The bacteriophage lambda. Cold Spring Harbor Laboratory Press, New York, p 737
Fukuda R, Iwakura Y, Ishihama A (1974) Heterogeneity of RNA polymerase in Escherichia coli, I. A new holoenzyme containing a new sigma factor. J Mol Biol 83:353–367
Glass RE, Jones ST, Ishihama A (1986) Genetic studies on the β subunit of Escherichia coli RNA polymerase, VII. RNA polymerase is a target for ppGpp. Mol Gen Genet 203:265–268
Greenblatt J, Li J (1981a) The nusA gene protein of Escherichia coli: Its identification and demonstration that it interacts with the gene N transcription anti-termination protein of bacteriophage lambda. J Mol Biol 147:11–23
Greenblatt J, Li J (1981b) Interaction of the sigma factor and the nusA gene protein of Eschericia coli with RNA polymerase in the initiation-termination cycle of transcription. Cell 24:421–428
Greenblatt J, Li J, Adhya S, Friedman DI, Baron LS, Redfield B, Kung H, Weissbach H (1980) Evidence that the L factor required for DNA-dependent in vitro synthesis of β-galactosidase is the Escherichia coli nusA gene protein. Proc Natl Acad Sci USA 77:1991–1994
Greenblatt J, McLimont M, Hanley S (1981) Termination of transcription by nusA gene protein of Escherichia coli. Nature 292:215–220
Hansen UM, McClure WR (1980) Role of the σ subunit of Escherichia coli RNA polymerase in initiation, II. Release of σ from ternary complexes. J Biol Chem 255:9564–9570
Imamoto F, Nakamura Y (1986) Escherichia coli proteins involved in regulation of transcription termination: Function, structure, and expression of the nusA and nusB genes. In: Ozeki H, Osawa S, Uchida H, Shimura Y, Ishihama A (eds) Genetic Systems. New York: Jpn Sci Soc Press/Elsevier, p 175–192
Ishihama A (1981) Subunit assembly of Escherichia coli RNA polymerase. Adv Biophys 14:1–35
Ishihama A (1986) Transcription signals and factors in Escherichia coli. In: Ozeki H, Osawa S, Uchida H, Shimura Y, Ishihama A (eds) Genetic Systems. New York: Jpn Sci Soc Press/Elsevier, p 163–173
Ishihama A, Fukuda R (1980) Autogenous and post-transcriptional regulation of RNA polymerase synthesis. Mol Cell Biochem 31:177–196
Ishihama A, Fukuda R, Kawakami K, Kajitani M, Enami M (1980) Transcriptional apparatus of Escherichia coli: Assembly of RNA polymerase and interplay with transcription factors. In: Osawa S, Ozeki H, Uchida H, Yura T (eds) Genetics and evolution of RNA polymerase, tRNA and ribosomes. Elsevier-North Holland, Amsterdam, p 105–115
Ishii S, Ihara M, Maekawa T, Nakamura Y, Uchida H, Imamoto F (1984a) The nucleotide sequence of the cloned nusA gene and its flanking region of Escherichia coli. Nucleic Acids Res 12:3333–3342
Ishii S, Kuroki K, Imamoto F (1984b) F-Met transfer RNA gene in the leader region of the nusA operon in Escherichia coli. Proc Natl Acad Sci USA 81:409–413
Iwakura Y, Ito K, Ishihama A (1974) Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet 133:1–23
Kassavatis K, Chamberlin M (1981) Pausing and termination of transcription within the early region of bacteriophage T7 DNA in vitro. J Biol Chem 256:2777–2786
Kajitani M, Ishihama A (1983a) Determination of the promoter strength in the mixed transcritpion system: Promoters of lactose, tryptophan and ribosomal protein L10 operons from Escherichia coli. Nucleic Acids Res 11:671–687
Kajitani M, Ishihama A (1983b) Determination of the promoter strength in the mixed transcription system: Promoters of ribosomal RNA, ribosomal protein S1 and recA operons from Escherichia coli. Nucleic Acids Res 11:3873–3888
Kajitani M, Ishihama A (1985) Promoter selectivity of Escherichia coli RNA polymerase: Differential stringent control of the multiple promoters from ribosomal RNA and protein operons. J Biol Chem 259:1951–1957
Kawakami K, Saitoh T, Ishihama A (1979) Biosynthesis of RNA polymerase in Escherichia coli, IX. Growth-dependent variations in the synthesis rate, content and distribution of RNA polymerase. Mol Gen Genet 174:107–116
Kingston RE, Chamberlin MJ (1981) Pausing and attenuation of in vitro transcription in the rrnB operon of Escherichia coli. Cell 27:523–531
Kung H, Weissbach H (1980) Further characterization of L factor, a protein required for β-galactosidase synthesis. Arch Biochem Biophys 201:544–550
Kung H, Spears C, Weissbach H (1975) Purification and properties of a soluble factor required for the deoxyribonucleic acid-directed in vitro synthesis of β-glactosidase. J Biol Chem 250:1556–1562
Kuroki K, Ishii S, Kano Y, Miyashita T, Nishi K, Imamoto F (1982) Involvement of the nusA and nusB gene products in transcription of Escherichia coli tryptophan operon in vitro. Mol Gen Genet 185:369–371
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laskey RA, Mills AD (1975) Quantitative film detection of 3H and 14C in polyacrylamide gels by flurography. Eur J Biochem 56:335–341
Mackeen LS, Diperi C, Schwartz I (1979) Reductive methylation of IF-3 and EF-Tu with [14C]formaldehyde and sodium cyanoborohydride. FEBS Lett 101:387–390
Nakamura Y, Mizusawa S (1985) In vivo evidence that the nusA and infB genes of Escherichia coli are part of the same multigene operon which encodes at least four proteins. EMBO J 4:527–532
Nakada N, Yoshinaga K, Ishihama A, Nagasawa-Fujimori H (1982) Noncoordinate synthesis of RNA polymerase ββ′ subunits in a temperature-sensitive β′-subunit mutant of Escherichia coli. Mol Gen Genet 188:173–178
Nene V, Glass RE (1983) Relaxed mutants of Escherichia coli RNA polymerase. FEBS Lett 153:307–310
Nomura T, Fujita N, Ishihama A (1985) Promoter selectivity of Escherichia coli RNA polymerase: Analysis of the promoter system of convergently-transcribed dnaQ-rnh genes. Nucleic Acids Res 13:7647–7661
Platt T (1981) Termination of transcription and its regulation in the tryptophan operon of Escherichia coli. Cell 24:10–23
Taketo M, Ishihama A, Kirschbaum JB (1976) Altered synthesis and stability of RNA polymerase holoenzyme subunits in mutants of Escherichia coli with mutations in the β or β′ subunit genes. Mol Gen Genet 147:139–143
Travers AA (1976) Modulation of RNA polymerase specificity by ppGpp. Mol Gen Genet 147:225–232
Travers AA, Burgess RR (1969) Cyclic re-use of the RNA polymerase sigma factor. Nature 222:537–540
Vogel HJ, Bonner DM (1969) Acetylornithinase of Escherichia coli: Partial purification and some properties. J Biol Chem 218:97–106
Yura T, Ishihama A (1979) Genetics of bacterial RNA polymerases. Annu Rev Genet 13:59–97
Author information
Authors and Affiliations
Additional information
Communicated by K. Isono
This is paper no. 14 in the series entitled “Biosynthesis of RNA polymerase in Escherichia coli”. Paper no. 13 is Nakada et al. (1982)
Rights and permissions
About this article
Cite this article
Ishihama, A., Honda, A., Nagasawa-Fujimori, H. et al. Multivalent regulation of the nusA operon of Escherichia coli . Mol Gen Genet 206, 185–191 (1987). https://doi.org/10.1007/BF00333573
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00333573