Abstract
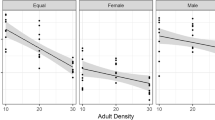
Life history theory predicts that larger propagules should be produced when the offspring are expected to experience intense competition. This study tested whether female cowpea weevils responded to high larval or adult density by producing larger eggs. In a splitbrood design I measured the effect of density experienced by females at their larval stage (1 vs. 4–6 larvae/cowpea) on the size of eggs produced just after emergence. The females were then kept either at low adult density (1 female+1 male per vial), or at high adult density (10 females+10 males) for 2 days, and tested for the effect of this adult density treatment on the size of eggs laid subsequently. I measured egg length and width, as well as the diameter of the entrance tunnel made by the larva, which can be regarded as a crude measure of larval size. Females that experienced high adult density subsequently laid slightly wider eggs than those kept at low density. This difference, albeit small (about 1–4% after correction for female weight and the effect of family, depending on the statistical model used), was statistically significant and robust to alterations of the statistical model. It may be a remnant of a larger plastic response of egg size to competition that has become eroded during many generations in high-density laboratory cultures. There was no difference in egg length or the diameter of the entrance tunnel. Eggs laid just after emergence by females reared at high larval density also tended to be wider than those produced by females that had no competitors. This effect was only marginally significant, however, and sensitive to the statistical model. Both egg length and width and the diameter of the entrance tunnel increased with female weight and decreased with female age. The tunnel diameter was positively correlated with both egg length and width, but the effect of width was larger.
Similar content being viewed by others
References
Begon M, Parker GA (1986) Should egg size and clutch size decrease with age? Oikos 47:293–302
Duellman WE, Trueb L (1986) Biology of amphibians. McGraw-Hill, New York
Fox CW (1993) The influence of maternal age and mating frequency on egg size and offspring performance in Callosobruchus maculatus (Coleoptera: Bruchidae). Oecologia 96:139–146
Giga DP, Smith RH (1985) Oviposition markers in Callosobruchus maculatus F. and Callosobruchus rhodesianus Pic. (Coleoptera, Bruchidae): asymmetry of interspecific responses. Agric Ecosyst Environ 12:229–233
Kawecki TJ (1993) Age and size at maturity in a patchy environment: fitness maximization versus evolutionary stability. Oikos 66:309–317
King DA (1990) The adaptive significance of tree height. Am Nat 135:809–828
Mäkela A (1985) Differential games in evolutionary theory: height growth strategies of trees. Theor Popul Biol 27:239–267
Maynard Smith J, Brown RL (1986) Competition and body size. Theor Popul Biol 30:166–179
McLachlan AJ (1989) Animal populations and extreme densities: size dimorphism by frequency-dependent selection in ephemeral habitats. Funct Ecol 3:633–637
Messina FJ (1991) Life-history variation in a seed beetle: adult egg-laying vs. larval competitive ability. Oecologia 85:447–455
Mirmirani M, Oster G (1978) Competition, kin selection, and evolutionary stable strategies. Theor Popul Biol 13:304–339
Møller H, Smith RH, Sibly RM (1989) Evolutionary demography of a bruchid beetle. I. Quantitative genetical analysis of the female life history. Funct Ecol 3:673–681
Orton RA, Sibly RM (1990) Egg size and growth rate in Theodoxus fluviatilis (L.). Funct Ecol 4:91–94
Parker GA, Begon M (1986) Optimal egg size and clustch size: effects of environment and maternal phenotype. Am Nat 128:573–592
Perrin N (1989) Population density and offspring size in the cladoceran Simocephalus vetulus (Muller). Funct Ecol 3:29–36
Reznick D, Endler JA (1982) The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36:160–177
Roff DA (1992) The evolutions of life histories. Chapman and Hall, New York
SAS Institute (1980) SAS/STAT user's guide, version 6 edn. SAS Institute, Cary
Sibly RM, Calow P (1983) An integrated approach to life-cycle evolution using selective landscapes. J Theor Biol 102:527–547
Sibly R, Calow P, Smith RH (1988) Optimal size in seasonal breeders. J Theor Biol 133:13–21
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Smith FE (1963) Population dynamics in Daphnia magna and a new model for population growth. Ecology 44:651–663
Sokal RR, Rohlf FJ (1969) Biometry Freeman, San Francisco
Stearns SC, (1992) The evolution of life histories. Oxford University Press, Oxford
Visser ME, Alphen JJM van, Nell HW (1990) Adaptive superparasitism and patch time allocation in solitary parasitoids: the influence of the number of parasitoids depleting a patch. Behaviour 114:21–36
Wilson K, Hill L (1989) Factors affecting egg maturation in the bean weevil Callosobruchus maculatus. Physiol Entomol 14:115–126
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawecki, T.J. Adaptive plasticity of egg size in response to competition in the cowpea weevil, Callosobruchus maculatus (Coleoptera: Bruchidae). Oecologia 102, 81–85 (1995). https://doi.org/10.1007/BF00333313
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00333313