Abstract
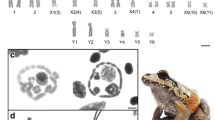
Cytogenetic data from 6 populations demonstrated unusual supernumerary chromosome variation in the primitive frog, Leiopelma hochstetteri. Frogs from the Coromandel Peninsula of the North Island of New Zealand averaged very high numbers of supernumerary chromosomes while individuals from other populations outside of the Coromandel region rarely had more than 1 distinctive supernumerary chromosome found only in females. The maximum number observed was 16 supernumeraries, present in 1 individual from Mt. Moehau. Supernumeraries showed meiotic instability as they failed to pair during prophase I in spermatocytes. In lampbrush preparations from oöcytes, supernumeraries appeared as univalents or as highly unusual stellate aggregations consisting of up to 7 chromosomes joined at their telomeres. Lateral loops on lampbrush supernumeraries indicated transcriptional activity. Contrary to a previous hypothesis, high supernumerary chromosome numbers in L. hochstetteri were not correlated with meiotic abnormalities. Neither were supernumerary chromosomes correlated with variations in heterochromatin distribution in the regular chromosomes. Rather, heterochromatin distribution was shown to vary geographically between populations. Sex determination in L. hochstetteri was found to be through a supernumerary, univalent W chromosome. Females in all populations invariably had 1 distinctive supernumerary chromosome not present in males. This chromosome could be distinguished from other supernumerary chromosomes by distinctive C-banding patterns and larger size. The W chromosome has undergone more rapid evolutionary change than the autosomes. Both telocentric and metacentric iso-chromosome forms were found in most populations. Heterochromatin distribution on the W chromosome varied between populations, from very little heterochromatin restricted to the centromere in Coromandel populations to an almost completely heterochromatic W chromosome among frogs from the East Cape region. In lampbrush preparations, the W chromosome was morphologically distinct from other supernumeraries. Loss of a Z chromosome leading to a univalent sex-determining W chromosome is difficult to explain through prevailing theories of sex-chromosome differentiation. The 0W♀/00♂ sex-determination system of L. hochstetteri appears to be unique among animals.
Similar content being viewed by others
References
Ashley T (1987) Meiotic behavior of sex chromosomes: what is normal? Chromosomes Today 9:184–195
Baker BS, Belote JH (1987) Sex determination and dosage compensation in Drosophila melanogaster. Annu Rev Genet 17:345–393
Baverstock PR, Watts CHS, Hogarth JT (1976) Heterochromatin variation in the Australian rodent Uromys caudimaculatus. Chromosoma 57:397–403
Bell BD (1982) The amphibian fauna of New Zealand. In: Newman DG (ed) New Zealand herpetology. New Zealand Wildlife Service, Wellington, pp 27–89
Bell BD (1985) Conservation Status of the endemic New Zealand frogs. In: Grigg G, Shine R, Ehrmann H (eds) Biology of Australasian frogs and reptiles. Surrey Beatty and Sons, Chipping Norton, pp 449–458
Bell BD (1986) The conservation status of New Zealand wildlife. Occ Publ NZ Wildlife Serv 12:1–103
Bogart JP (1981) Chromosome studies in Sminthillus from Cuba and Eleutherodactylus from Cuba and Puerto Rico (Anura, Leptodactylidae). Life Sci Contrib R Ontario Museum 129:1–22
Boyes JW (1967) The cytology of muscoid flies. In: Wright JW, Pal R (eds) Genetics of insect vectors of disease. Elsevier, Amsterdam, pp 371–384
Boyes JW, Van Brink JM (1967) Chromosomes of syrphidae III. Karyotypes of some species in the tribes Milesiini and Myoleptini. Chromosoma 22:417–455
Bull JJ (1983) Evolution of sex determining mechanisms. Benjamin/ Cumming, Menlo Park, California
Callan HG (1986) Lampbrush chromosomes. Springer, Berlin Heidelberg New York
Callan HG, Lloyd L (1960) Lampbrush chromosomes of crested newts Triturus cristatus Laurenti. Philos Trans R Soc London Ser B 243:135–219
Callan HG, Gall JG, Berg CA (1987) The lampbrush chromosomes of Xenopus laevis: preparation, identification and distribution of 5s DNA sequences. Chromosoma 95:236–250
Chang CY, Witschi E (1955) Breeding of sex-reversed males of Xenopus laevis Daudin. Proc Soc Exp Biol Med 89:150–152
Charlesworth B (1978) Model for the evolution of Y chromosomes and dosage compensation. Proc Natl Acad Sci USA 75:5618–5622
Charlesworth B, Coyne JA, Barton NH (1987) The relative rates of evolution of sex chromosomes and autosomes. Am Nat 130:113–146
Dev VG, Miller DA, Tantravani RR, Schreck RR, Roderick TH, Erlanger BF, Miller OJ (1976) Chromosome markers in Mus musculus: differences in C-banding between the subspecies M. m. musculus and M. m. molossinus. Chromosoma 53:335–344
Duellman WE, Trueb L (1986) The biology of amphibians. McGraw-Hill, New York
Felsenstein J (1974) The evolutionary advantage of recombination. Genetics 78:737–756
Franco MG, Rubini PG, Vecchi M (1982) Sex determinants and their distribution in various populations of Musca domestica L. of western Europe. Genet Res 40:279–293
Giorgi F, Galleni L (1972) The lampbrush chromosomes of Rana esculenta L. (Amphibia-Anura). Caryologia 25:107–123
Goodfellow PN, Davies KE, Ropers HH (1985) Report of the committee on the genetic constitution of the human X and Y chromosome. Cytogenet Cell Genet 40:296–352
Goodfellow PN, Goodfellow PJ, Pym B, Banting C, Pritchard C, Darling SM (1987) Genes on the human Y chromosome. In: Hazeltine FP, McClure ME, Goldberg EH (eds) Genetic markers of sex determination. Plenum Press, New York, pp 99–111
Green DM (1985) Differentiation in amount of centromeric heterochromatin between subspecies of the red-legged frog, Rana aurora. Copeia 1985:1071–1074
Green DM (1988) Heteromorphic sex-chromosomes in the rare and primitive frog Leiopelma hamiltoni from New Zealand. J Hered 79:165–169
Green DM, Sharbel TF (1988) Comparative cytogenetics of the primitive frog Leiopelma archeyi (Amphibia, Anura). Cytogenet Cell Genet, in press
Green DM, Bogart JP, Anthony EH, Genner DL (1980) An interactive, microcomputer based karyotype analysis system for phylogenetic cytotaxonomy. Comput Biol Med 10:219–227
Green DM, Kezer J, Nussbaum RA (1984a) Triploidy in Hochstetter's frog Leiopelma hochstetteri from New Zealand. NZ J Zool 11:457–461
Green DM, Myers PZ, Reyna DL (1984b) CHROMPAC III: an improved package for micro-computer assisted analysis of karyotypes. J Hered 75:143
Green DM, Kezer J, Nussbaum RA (1987) Supernumerary chromosome variation and heterochromatin distribution in the endemic New Zealand frog, Leiopelma hochstetteri. Chromosoma 95:339–344
Harvey AW, Hewitt GM (1979) B-chromosomes slow development in a grasshopper. Heredity 42:397–401
Hewitt GM, East TM, Shaw MW (1987) Sperm dysfunction produced by B-chromosomes in the grasshopper Myrmeleotettix maculatus. Heredity 58:59–68
Hodgkin J (1987) Sex determination and dosage compensation in Caenorabditis elegans. Annu Rev Genet 21:133–154
Hsu TC (1981) Polymorphism in human acrocentric chromosomes and the silver staining method for nucleolus organizer regions. Karyogram 7:45
Jones RN, Rees H (1982) B-Chromosomes. Academic Press, New York, p 266
Kezer J, Sessions SK (1979) Chromosome variation in the plethodontid salamander. Aneides ferreus. Chromosoma 71:65–80
Kezer J, Léon PE, Sessions SK (1980) Structural differentiation of the meiotic and mitotic chromosomes of the salamander Ambystoma macrodactylum. Chromosoma 81:177–197
Levan A, Fredga D, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220
Milani R, Rubini PG, Franco MG (1967) Sex determination in the housefly. Genetica Agraria 21:385–411
Morescalchi A (1967) The karyotype of two specimens of Leiopelma hochstetteri Fitz. (Amphibia Salientia). Caryologia 21:37–46
Muller HJ (1964) The relation of recombination to mutational advance. Mutat Res 43:165–229
Page DC, Mosher R, Simpson EM, Fisher EMC, Mardon G, Pollack J, McGillivray B, dela Chappelle A, Brown LG (1987) The sex-determining region of the human Y chromosome encodes a finger protein. Cell 51:1091–1104
Panse K (1942) Sur la digametie du crapaud hermaphrodite. Rev Suisse Zool 49:185–189
Parker JS, Ainsworth CC, Taylor S (1981) The B-chromosome system of Hypochoeris maculata II. B-effects on meiotic Achromosome behaviour. Chromosoma 67:123–143
Patton JL (1977) B-chromosome systems in the pocket mouse, Perognathus baileyi: meiosis and C-band studies. Chromosoma 60:1–14
Peeters JP, Griffiths AJF, Wilkes G (1985) In vivo karyotypic modifications following spontaneous cell fusion in maize (Zea mays L.). Can J Genet Cytol 27:580–585
Rice WR (1987) Genetic hitchhiking and the evolution of reduced gene activity of the Y sex chromosome. Genetics 116:161–167
Sannomiya M (1974) Cytogenetic studies on natural populations of grasshoppers with special reference to B chromosomes. I. Gonista bicolor. Heredity 32:251–265
Schmid M (1978) Chromosome banding in amphibia I. Constitutive heterochromatin and nucleolus organizer regions in Bufo and Hyla. Chromosoma 66:361–388
Schmid M (1983) Evolution of sex chromosomes and heterogametic systems in Amphibia. Differentiation 23 (Suppl):S13-S22
Sessions SK (1984) Cytogenetics and evolution in salamanders. Ph D dissertation. University of California, Berkeley
Stephenson EM, Robinson ES, Stephenson NG (1972) Karyotype variation within the genus Leiopelma (Amphibia: Anura). Can J Genet Cytol 14:691–702
Stephenson EM, Robinson ES, Stephenson NG (1974) Inter-specific relationships of Leiopelma (Amphibia: Anura). Further karyological evidence. Experientia 30:1248–1250
Suja JA, Gosalvez J, Lopez-Fernande C, Rufas JS (1986) A cytogenetic analysis in Psophus stridulus (L.) (Orthoptera: Acrididae): B-chromosomes and abnormal spermatid nuclei. Genetica 70:217–224
Weissenbach J (1987) A molecular analysis of the human Y chromosome. Chromosomes Today 9:165–174
Worthy TH (1987) Osteology of Leiopelma (Amphibia: Leiopelmatidae) and descriptions of three new subfossil Leiopelma species. J R Soc NZ 17:201–251
Yanagimachi R (1961) Studies on the sexual organization of the Rhizocephala III. The mode of sex-determination in Peltogasterella. Biol Bull 120:272–283
Yoshida TH, Sagai T (1975) Variation of C-bands in the chromosomes of several subspecies of Rattus rattus. Chromosoma 50:283–300
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Green, D.M. Cytogenetics of the endemic New Zealand frog, Leiopelma hochstetteri: extraordinary supernumerary chromosome variation and a unique sex-chromosome system. Chromosoma 97, 55–70 (1988). https://doi.org/10.1007/BF00331795
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00331795