Summary
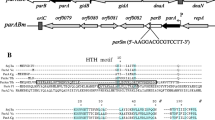
Mutations at the cpxA locus of Escherichia coli K-12 affect cellular processes that are not otherwise related. We have now determined the physical and genetic structure of the E. coli chromosome in the region of cpxA (87.5 min). Our results indicate that cpxA is a single gene. Previous studies showed cpxA to be linked to tpiA. We therefore isolated two tpiA + recombinant plasmids, pRA200 and pRA300, from EcoRI and BamHI digests of F′133, respectively. By genetic complementation or enzyme overproduction, the 9.5 kb EcoRI fragment in pRA200 was shown to include glpK, tpiA and cdh. The 13.6 kb BamHI fragment of pRA300 lacks glpK, but includes tpiA, pfkA and cpxA. Neither fragment complemented a deletion of the rha operon. These data indicate the chromosomal gene order: 87 min-rha-cpxA-pfkA-cdh-tpiA-glpK-88 min. The EcoRI and BamHI fragments overlap in an interval corresponding to about 8.2 kb of DNA. The total region of the E. coli K12 chromosome covered by the two fragments is about 15 kb. A terminal 2 kb EcoRI-BamHI fragment from pRA300 complemented the chromosomal cpxA2[Ts] allele with respect to isoleucine and valine synthesis, RNA bacteriophage sensitivity and surface exclusion in Hfr strains, and envelope protein composition. Complementation occurred when the fragment was subcloned in pBR325 but not when it was subcloned in pBR322, suggesting that the 2 kb fragment lacks expression sequences that are supplied by cat (chloramphenicol acetyltransferase gene) expression sequences of pBR325. The cpxA locus on the E. coli chromosome was established with respect to two chromosomal Tn10 insertions by a combination of genetic and physical analyses. The locus established by those analyses was consistent with the location of the 2 kb EcoRI-BamHI fragment in the physical map of the region. Physical analyses of (rha-pfkA) and (rha-tpiA) deletion strains showed that they lack cpxA and surrounding genes. Since these strains were viable, cpxA is not essential under all growth conditions.
Similar content being viewed by others
References
Albin R, Silverman P (1984) Identification of the Escherichia coli K-12 cpxA locus as a single gene: construction and analysis of biologically-active cpxA gene fusions. Mol Gen Genet 197:272–279
Armstrong K, Fan D (1976) Essential genes in the metB-malB region of Escherichia coli K-12. J Bacteriol 126:48–55
Babul J (1978) Phosphor fructokinases from Escherichia coli. J Biol Chem 253:4350–4355
Bachmann B (1983) Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev 47:180–230
Cohen S, Chang A, Hsu L (1972) Non-chromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci USA 69:2110–2114
Conrad C, Stearns G, Prater W, Rheiner J, Johnson J (1984) Characterization of a glpK transducing phage. Mol Gen Genet 193:376–378
Cuozzo M, Silverman P, Minkley E, Jr (1984) Overproduction in Escherichia coli K-12 and purification of the TraJ protein encoded by the conjugative plasmid F. J Biol Chem 258:6659–6666
Eoyang L, Silverman P (1984) Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K-12. J Bacteriol 157:184–189
Fowler T, Taylor L, Thompson R (1983) The control region of the F-plasmid transfer operon: DNA sequence of the traJ and traY genes and characterization of the traY-Z promoter. Gene 26:79–89
Gaffney D, Skurray R, Willetts N (1983) Regulation of the F connjugation genes studies by hybridization and tra-lacZ fusion. J Mol Biol 168:103–122
Hansen J, Olsen R (1978) Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids PMG1 and PMG5. J Bacteriol 135:227–238
Humphreys G, Willshaw G, Anderson E (1975) A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta 383:457–463
Jorgensen R, Berg D, Allet B, Reznikoff W (1979) Restriction enzyme cleavage map of Tn10, a transposon which encodes tetracycline resistance. J Bacteriol 137:681–685
Maniatis T, Fritsch E, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, New York, pp 366–369
McDonell M, Simon M, Studier FW (1977) Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol 110:119–146
McEwen J, Silverman P (1980a) Chromosomal mutations of Escherichia coli K-12 that alter the expression of conjugative plasmid function. Proc Natl Acad Sci USA 77:513–517
McEwen J, Silverman P (1980b) Genetic analysis of Escherichia coli K-12 chromosomal mutants defective in the expression of F-plasmid functions: identification of genes cpxA and cpxB. J Bacteriol 144:60–67
McEwen J, Silverman P (1980c) Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in isoleucine and valine synthesis. J Bacteriol 144:68–73
McEwen J, Silverman P (1982) Mutations in genes cpxA and cpxB alter the protein composition of the Escherichia coli K-12 inner and outer membranes. J Bacteriol 144:1553–1559
McEwen J, Sambucetti L, Silverman P (1983) Synthesis and translocation of outer membrane proteins in cpxA cpxB mutants of Escherichia coli K-12. J Bacteriol 154:375–382
Morris J, Newman E (1980) Map location of the ssd mutation in Escherichia coli K-12. J Bacteriol 143:1504–1505
Newman E, Malik N, Walker C (1982) L-serine degradation in Escherichia coli K-12: directly isolated ssd mutants and their intragenic revertants. J Bacteriol 150:710–715
Pahel G, Bloom F, Tyler B (1979) Deletion mapping of the polA-metB region of the Escherichia coli chromosome. J Bacteriol 138:653–656
Pouyssegur J, Lagarde A (1973) Systeine de transport du 2-ceto-3-desoxygluconate chez E. coli K-12: localisation d'un gene du structure et de son operateur. Mol Gen Genet 121:163–180
Pouyssegur J, Stoeber F (1974) Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdgR regulon. J Bacteriol 117:641–651
Prentki P, Karch F, Iida S, Meyer J (1981) The plasmid cloning vector pBR325 contains a 482 base-pair-long inverted duplication. Gene 14:289–299
Rigby P, Dieckmann M, Rhodes C, Berg P (1977) Labeling deoxyribonucleic acid to high specific activity in vitro by nick-translation. J Mol Biol 113:237–251
Sambucetti L, Eoyang L, Silverman P (1982) Cellular control of conjugation in Escherichia coli K-12: effect of chromosomal mutations on F-plasmid gene expression. J Mol Biol 161:13–31
Shimosaka M, Fukuda Y, Murata K, Kimura A (1982) Application of hybrid plasmids carrying glycolysis genes to ATP production by Escherichia coli. J Bacteriol 152:98–103
Silverman P (1982) Gene cpxA is a new addition to the linkage map of Escherichia coli K-12. J Bacteriol 150:425–428
Sutton A, Newman T, McEwen J, Silverman P, Freundlich M (1982) Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a posttranslational defect in acetohydroxyacid synthase I function in vivo. J Bacteriol 151:976–982
Thomson J, Gerstenberger P, Goldberg D, Gociar E, Silva A, Fraenkel D (1979) ColE1 hybrid plasmids for Escherichia coli genes of glycolysis and the hexose monophosphate shunt. J Bacteriol 137:502–506
Touati D (1983) Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol 155:1078–1087
Umbarger HE (1978) Amino acid biosynthesis and its regulation. Annu Rev Biochem 47:533–606
Vogel H, Bonner D (1956) Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97–106
Willetts N (1977) The transcriptional control of fertility in F-like plasmids. J Mol Biol 112:141–148
Womble D, Taylor D, Rownd R (1977) Method for obtaining more accurate covalently closed circular plasmid-to-chromosome ratios from bacterial lysates by dye-buoyant density centrifugation. J Bacteriol 130:148–153
Author information
Authors and Affiliations
Additional information
Communicated by W. Arber
Rights and permissions
About this article
Cite this article
Albin, R., Silverman, P.M. Physical and genetic structure of the glpK-cpxA interval of the Escherichia coli K-12 chromosome. Mol Gen Genet 197, 261–271 (1984). https://doi.org/10.1007/BF00330972
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00330972