Abstract
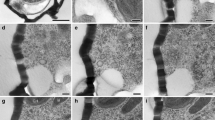
Exposure of Nyctotherus ovalis to low temperatures or vinblastine caused similar reactions of “classes” of microtubules (mt) present in the mitotic micronucleus of this ciliate towards both treatments. However, differences of sensitivity between certain “classes” of mt at individual mitotic stages exist. Unlike the kinetochore mt (kmt) of most other eukaryotic cells, kmt in Nyctotherus completely disassemble after incubation at 6–8 ° C (60 min) and most disappear after prolonged exposure to vinblastine (10−5 M, 16 h). The depolymerization of kmt causes the collapse of the spindle and a dislocation of chromosomes at metaphase, yet the reduced number of kmt after vinblastine-treatment still allows an alignment of composite complexes at the spindle equator. The data suggest that three individual sets of mt exist in the interpolar spindle region during ana- and telophase: 1) interpolar mt (int mt), which are assembled during anaphase, are cold- and vinblastine sensitive; 2) manchette mt (ma mt), which are first observed underneath the nuclear envelope during mid-anaphase, are cold-stable and insensitive to vinblastine treatment (10−5 M); after prolonged treatment (16 h) they form spiral structures; 3) stembody mt (st mt), comprising the interpolar region of the nucleus during telophase, are cold- and vinblastine insensitive. Paracrystalline structures resembling a stembody are formed in telophase-like division stages after prolonged vinblastine exposure (16 h, 10−5 M). Since kmt and int mt possess the same sensitivity under depolymerizing conditions, they probably have a similar composition. Thus the idea that the int mt in this organism arise by elongation of kmt is supported. However, st mt apparently do not originate from an extension of preexistent int mt, but appear to represent a new set of stable mt. This is emphasized not only by their greater stability compared to the int mt but also by the distribution of cold-stable mt in late anaphase micronuclei. The ma mt may be an intermediary step in formation of st mt since their stability resembles that of the st mt. A comparison of the substructure of vinblastine-induced paracrystals in Nyctotherus with those observed in in vitro systems with known composition suggests that a turnover of MAPs may be responsible for the different stability of mt and thus could specify and regulate mt sensitivity and function. Another organelle, possibly involved in conferring stability to mt, is the nuclear membrane. The assumption that the nuclear envelope possesses an intrinsic property to nucleate mt and thus aid in the alignment of mt is supported.
Similar content being viewed by others
References
Bajer, A.S., Molé-Bajer, J.: Spindle dynamics and chromosome movement. Int. Rev. Cytol. Suppl. 2 (1972)
Behnke, O., Forer, A.: Evidence for four classes of microtubules in individual cells. J. Cell Sci. 2, 169–192 (1967)
Brinkley, B.R.: Cold-labile and cold-stable microtubules in the mitotic spindle of mammalian cells. Annals N.Y. Acad. Sci. 253, 428–439 (1975)
DeMey, J., Moeremans, M., Geuens, G., Nuydens, R., Van Belle, H., De Brabander, M.: Immunocytochemical evidence for the association of calmodulin with microtubules in the mitotic apparatus. In: Microtubules and microtubule inhibitors (M. De Brabander and J. DeMey, eds.) pp. 227–242. New York: Elsevier Press 1980
Cohen, J., Beisson, J., Tucker, J.B.: Abnormal microtubule deployment during defective macronuclear division in a Paramecium mutant. J. Cell Sci. 44, 153–167 (1980)
Conolly, J.A., Kalnius, U.I.: Tau and HMW microtubule-associated proteins have different microtubule binding sites in vitro. Eur. J. Cell Biol. 21, 296–300 (1980)
Dustin, P.: Microtubules. Heidelberg New York: Springer 1978
Eichenlaub-Ritter, U., Ruthmann, A.: Holokinetic composite chromosomes with “diffuse” kinetochores in the micronuclear mitosis of a heterotrichous ciliate. Chromosoma (Berl.) 84, 701–716 (1982)
Eichenlaub-Ritter, U.: Micronuclear mitosis in the ciliate Nyctotherus ovalis Leidy: an unusual chromosome arrangement and kinetochore structure and its implications for spindle structure and function. In: Microtubules in microorganisms (P. Cappuccinelli and N.R. Morris, eds.) New York: Marcel Dekker, Inc. (in press, 1982)
Fujiwara, K., Tilney, L.G.: Substructural analysis of the microtubule and its polymorphic forms. Annals N.Y. Acad. Sci. 253, 27–50 (1975)
Haskins, K.M., Donoso, J.A., Himes, R.H.: Spirals and paracrystals induced by vinca alkaloids: evidence that microtubule associated proteins act as polycations. J. Cell Sci. 47, 237–247 (1981)
Hepler, P.K.: Membranes in the mitotic apparatus of barley cells. J. Cell Biol. 86, 490–499 (1980)
Hepler, P.K., Wich, S.M., Wolniak, S.M.: The structure and role of membranes in the mitotic apparatus. In: International cell biology 1980–1981 (H.G. Schweiger, ed.) pp. 673–686. Berlin Heidelberg New York: Springer 1981
Jockusch, B.M., Blessing, J.: Structural differences in vinblastine-induced aggregates of acidic proteins. Exp. Cell Res. 69, 465–467 (1971)
Jones, J.C.R., Tucker, J.B.: Microtubule-organizing centres and assembly of the double-spiral microtubule pattern in certain heliozoan axonemes. J. Cell Sci. 50, 259–280 (1981)
LaFountain, J.R., Davidson, L.A.: An analysis of spindle ultrastructure during prometaphase and metaphase of micronuclear division in Tetrahymena. Chromosoma (Berl.) 75, 293–308 (1979)
Lambert, A,-M., Bajer, A.S.: Microtubule distribution and reversible arrest of chromosome movements induced by low temperature. Cytobiol. 15, 1–23 (1977)
Lambert, A.-M.: The role of chromosomes in anaphase trigger and nuclear envelope activity in spindle formation. Chromosoma 76, 295–308 (1980)
Lambert, A.-M., Berner-Schmidt, A.C.: Chromosome dynamics and spindle structure during arrest of anaphase induced by colchicine, vinblastine and cold-treatments. 2nd. Int. Symp. Microtubules and microtubule inhibitors, Abstr. 65, Beerse 1980
Luduena, R.F., Fellous, A., Frencon, J., Nunez, J., McManus, L.: Effect of tau on vinblastine-induced aggregation of tubulin. J. Cell Biol. 89, 680–683 (1981)
Margolis, R.L., Wilson, L.: Mitotic mechanism based on intrinsic microtubule behavior. Nature (Lond.) 272 (5452), 450–452 (1978)
Murphy, D.B., Johnson, K.A., Borisy, G.G.: Role of tubulin-associated proteins in microtubule nucleation and elongation. J. Mol. Biol. 117, 33–52 (1977)
Oppenheim, D.S., Hauschka, B.T., McIntosh, J.R.: Anaphase motions in dilute colchicine. Evidence for two phases in chromosome segregation. Exp. Cell Res. 79, 95–105 (1973)
Rieder, C.L.: The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma (Berl.) 84, 145–158 (1981)
Roos, U.-P.: Light and electron microscopy of rat Kangaroo cells in mitosis. III Pattern of chromosome behavior during prometaphase. Chromosoma (Berl.) 54, 363–385 (1976)
Roth, L.E.: Electron microscopy of mitosis in amoebae. III. Cold and urea treatments: A basis for test of direct effects of mitotic inhibitors on microtubule formation. J. Cell Biol. 34, 47–59 (1967)
Salmon, E.D., Goode, D., Magel, T.K., Bonar, D.B.: Pressure-induced depolymerization of spindle microtubules. III Differential stability in HeLa cells. J. Cell Biol. 69, 443–454 (1976)
Salmon, E.D., Begg, D.A.: Functional implications of cold-stable microtubules in kinetochore fibers of insect spermatocytes during anaphase. J. Cell Biol. 85, 853–865 (1980)
Starling, D.: Two ultrastructurally distinct tubulin paracrystals induced in sea-urchin eggs by vinblastine sulphate. J. Cell Sci. 20, 79–90 (1976)
Tucker, J.B.: Microtubule organization in protozoa. In: Microtubules in micro-organisms (P. Cappuccinelli and N.R. Morris, eds.) New York: Marcel Dekker, Inc. (in press, 1982)
Wilson, L.: Interaction of drugs with microtubule proteins. Fed. Proc. 33, 156–167 (1974)
Wolfe, J., Hunter, B., Adair, W.S.: A cytological study of micronuclear elongation during conjugation in Tetrahymena. Chromosoma (Berl.) 55, 289–308 (1976)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eichenlaub-Ritter, U., Ruthmann, A. Evidence for three “classes” of microtubules in the interpolar space of the mitotic micronucleus of a ciliate and the participation of the nuclear envelope in conferring stability to microtubules. Chromosoma 85, 687–706 (1982). https://doi.org/10.1007/BF00330781
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00330781