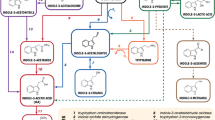
Summary
The genetic organization of functions responsible for mannityl opine catabolism of the Ti plasmid of Agrobacterium tumefaciens strain 15955 was investigated. A partial HindIII digest of pTi15955 was cloned into a broad host range cosmid and the clones obtained were tested for ability to confer mannityl opine degradation upon Agrobacterium. Inserts containing genes for catabolism of mannopinic acid, mannopine, agropine, and agropinic acid were obtained, spanning a segment of 43 kb on the Ti plasmid. Two clones conferring upon Agrobacterium the ability to catabolize the mannityl opines were mobilized to several Rhizobium sp., to Pseudomonas putida and P. fluorescens and to Escherichia coli. The catabolic functions were phenotypically expressed in all Rhizobium sp. tested, and in P. fluorescens, but not in P. putida or in E. coli.
Similar content being viewed by others
References
Casse F, Boucher C, Julliot JS, Michel M, Denarié J (1979) Identification and characterization of large plasmids in Rhizobium meliloti using agarose gel electrophoresis. J Gen Microbiol 113:229–242
Chilton WS, Chilton M-D (1984) Mannityl opine analogs allow isolation of catabolic pathway regulatory mutants. J Bacteriol 158:650–658
Chilton WS, Tempé J, Matzke M, Chilton M-D (1984) Succinamopine: a new crown gall opine. J Bacteriol 157:357–362
Coxon TD, Davies AMC, Fenwick GR, Self R, Firmin JL, Lipkin D, Janes NF (1980) Agropine, a new amino acid derivative from crown-gall tumors. Tetrahedron Lett 21:495–498
Darzins A, Chakrabarty AM (1984) Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol 159:9–18
De Greve H, Decraemer H, Seurinck J, Van Montagu M, Schell J (1981) The functional organization of the Agrobacterium tumefaciens plasmid pTiB653. Plasmid 6:235–248
Depicker A, De Wilde M, De Vos G, De Vos M, Van Montagu M, Schell J (1980) Molecular cloning of overlapping segments of the nopaline Ti plasmid pTIC58 as a means to restriction endonuclease mapping. Plasmid 3:193–211
Dessaux Y, Guyon P, Farrand SK, Petit A, Tempé J (1986) Agrobacterium Ti and Ri plasmids specify enzymic lactonization of mannopine to agropine. J Gen Microbiol 132:2549–2559
De Vos G, De Beuckeleer M, Van Montagu M, Schell J (1981) Restriction endonuclease mapping of the tumor inducing plasmid pTiACH5 of Agrobacterium tumefaciens. Plasmid 6:249–253
Ditta G, Stanfield S, Corbin D, Helsinski DR (1980) Broad host range DNA cloning system for gram-negative bacteria: contruction of a gene bank of R. meliloti Proc Natl Acad Sci USA 77:7347–7351
Ellis JG, Murphy PJ (1981) Four new opines from crown gall tumors — their detection and properties. Mol Gen Genet 181:36–43
Ellis JG, Ryder MH, Tate ME (1984) Agrobacterium tumefaciens TR-DNA encodes a pathway for agropine biosynthesis. Mol Gen Genet 195:466–473
Farrand SK, Kado CI, Ireland CR (1981) Suppression of tumorigenicity by the IncW R plasmid pSa in Agrobacterium tumefaciens. Mol Gen Genet 181:44–51
Farrand SK, Slota JE, Shim J-S, Kerr A (1985) Tn5 insertions in the agrocin 84 plasmid: the conjugal nature of pAgK84 and the locations of determinants for transfer and agrocin 84 production. Plasmid 13:106–117
Firmin JL, Fenwick GR (1978) Agropine: a major new plasmid-determined metabolite in crown gall tumours. Nature 276:842–844
Gelvin SB (1984) Plant tumorigenesis. In: Kosuge T, Nester EW (eds) Plant microbe interactions: molecular and genetic perspectives, vol 1. Macmillian Publishing, New York, p 343–377
Gelvin SB, Gordon MP, Nester EW, Aronson AI (1981) Transcription of the Agrobacterium Ti plasmid in the bacterium and in crown gall tumors. Plasmid 6:17–29
Gheysen G, Dhaese P, Van Montagu M, Schell J (1985) DNA flux across genetic barriers: the crown gall phenomenon. In: Hohn B, Dennis ES (eds) Plant gene research: genetic flux in plant. Springer, Wien, New York, p 11–47
Guyon P, Chilton M-D, Petit A, Tempé J (1980) Agropine in “null-type” tumors: evidence for generality of the opine concept. Proc Natl Acad Sci USA 77:2693–2697
Hille J, Hoekema A, Hooykaas PJJ, Schilperoort RA (1984) Gene organization of the Ti-plasmid. In: Verma DPS, Hohn T (eds) Genes involved in microbe-plant interactions. Springer, Wien, New York, p 287–309
Holsters M, De Waele D, Depicker A, Messens E, Van Montagu M, Schell J (1978) Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet 163:181–187
Holsters M, Silva B, Van Vliet F, Genetello C, De Block M, Dhaese P, Depicker A, Inze D, Engler G, Villaroel R, Van Montagu M, Schell J (1980) The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid 3:212–230
Hooykaas PJJ (1983) Plasmid genes essential for the interactions of Agrobacteria and Rhizobia with plant cells. In: Pühler A (ed) Molecular genetics of the bacteria-plant interaction. Springer, Berlin, Heidelberg, p 229–239
Hooykaas PJJ, Klapwijk PM, Nuti MP, Schilperoort RA, Korsch A (1977) Transfer of the Agrobacterium tumefaciens Ti plasmid to avirulent agrobacteria and toe Rhizobium ex planta. J Gen Microbiol 98:477–484
Kiss GB, Vincze E, Kalman Z, Forrai T, Kondorosi A (1979) Genetic and biochemical analysis of mutants affected in nitrate reduction in Rhizobium meliloti. J Gen Microbiol 113:105–118
Knauf VC, Nester EW (1982) Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid 8:45–54
Mandel M, Higa J (1970) Calcium-dependent bacteriophage DNA infection J Mol Biol 53:159–162
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Nester EW, Gordon MP, Amasino RM, Yanofsky MF (1984) Crown gall: a molecular and physiological analysis. Annu Rev Plant Physiol 35:387–413
Olexy WM, Bird T, Grieble HG, Farrand SK (1979) Hospital isolates of Serratia marcescens transferring ampicillin, carbenicillin and gentamicin resistance to other gram-negative bacteria including Pseudomonas aeruginosa. Antimicrob Agents Chemother 15:93–100
Petit A, Tempé J (1978) Isolation of Agrobacterium Ti plasmid regulatory mutants. Mol Gen Genet 167:147–155
Petit A, Tempé J (1985) The function of T-DNA in Nature. In: Van Vloten-Doting L, Groot GSP, Hall TC (eds) Molecular form and function of the plant genome, Plenum Press, New York, p 625–636
Petit A, David C, Dahl GA, Ellis JG, Guyon P, Casse-Delbart F, Tempé J (1983) Further extension of the opine concept: plasmids in Agrobacterium rhizogenes cooperate for opine degradation. Mol Gen Genet 190:204–214
Pühler A (1983) Molecular genetics of the bacteria-plant interactions. Springer, Berlin, Heidelberg, New York, Tokyo
Stachel SE, An G, Flores C, Nester EW (1985) A Tn3lacZ transposon for the random generation of β-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J 4:891–898
Tate ME, Ellis JG, Kerr A, Tempé J, Murray KE, Shaw KJ (1982) Agropine: a revised structure. Carbohydr Res 104:105–120
Tempé J, Guyon P, Tepfer DA, Petit A (1979) The role of opines in the ecology of the Ti plasmids of Agrobacterium. In: Timmis KN, Pühler A (eds) Plasmids of medical, environmental and commercial importance. Elsevier North Holland Biomedical Press, Amsterdam, New York, Oxford, p 353–363
Tepfer DA, Tempé J (1981) Production d'agropine par des racines formées sous l'action d'Agrobacterium rhizogenes. CR Séances Acad Sci Paris [III] 292:153–156
Trevelyan WE, Procter DP, Harrisson JP (1950) Detection of sugars on paper chromatography. Nature 166:444–445
Van Montagu M, Schell J (1979) The plasmids of Agrobacterium. In: Timmis KN, Pühler A (eds) Plasmids of medical, environmental and commercial importance, Elsevier North Holland Biomedical Press, Amsterdam, New York, Oxford, p 71–95
Verma DPS, Hohn T (1984) Plant gene research: genes involved in microbe plant interactions, Springer, Wien, New York
Author information
Authors and Affiliations
Additional information
Communicated by G. Schell
Rights and permissions
About this article
Cite this article
Dessaux, Y., Tempé, J. & Farrand, S.K. Genetic analysis of mannityl opine catabolism in octopine-type Agrobacterium tumefaciens strain 15955. Mole Gen Genet 208, 301–308 (1987). https://doi.org/10.1007/BF00330457
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00330457