Summary
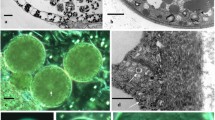
The new genus Inodosporus was erected to accept I. spraguei, a new species having eight sporoblasts per pansporoblast with each subsequent spore possessing three or four basal spore-tails and one branched apical one. It is primarily by the apical tail that the species is separated from the only other recognized species, I. octospora (Henneguy, 1892) comb. n., formerly Thelohania octospora.
Spore-tails of I. spraguei are membranous channels which originate within differentiating pansporoblasts during genesis of sporonts into sporoblasts. During the switch from vegetative to spore-forming development, cytoplasmic constituents of I. spraguei segregate into two distinctive domains for which we originate the terms “pansporoblast-determinate area” (PDA) and “sporont-determinate area” (SDA). Membrane channels, which form spore-tails, develop within the PDA.
The following observations indicate that the tails of I. spraguei are continuous with the outer pansporoblast envelope: lanthanum marker readily penetrates pansporoblasts and localizes in channels, in spore-tail attachment points, and between extra-sporoblast membrane and sporoblasts; a positive reaction for adenosine triphosphatase product accumulates within spore-tails at their sites of attachment to sporoblasts; and spore-tails occasionally remain attached to pansporoblast envelopes after mechanical disruption.
An extensive PAS-positive glycocalyx-like material is found within newly developing pansporoblasts. This observation, plus the presence of an apparent adenosine triphosphatase system on pansporoblast membranes, indicates that the pansporoblast may serve as a molecular or ion transport system during initial phases of sporont differentiation.
Inodosporus spraguei infects each muscle fiber completely until filaments are destroyed, and infections are spread throughout the animal until most fibers are infected. Curiously, uninfected muscle cells seldom show serious pathological changes caused by massive infections of neighboring cells.
Similar content being viewed by others
References
Bennett, H. S.: The cell surface: Movements and recombinations. In: Handbook of molecular cytology. A. Lima-De-Faria, ed. New York: American Elsevier Publishing Comp., Inc. 1969
Codreanu, R.: On the occurrence of spore or sporont appendages in the Microsporidia and their taxonomic significance. In: Proc. 1st Intern. Cong. Parasitol. A. Corradetti, ed. New York: Pergamon Press 1966
Codreanu, R., Vávra, J.: The structure and ultrastructure of the microsporidian Telomyxa glugeiformis Leger and Hesse, 1910, parasite of Ephemera danica (Müll.). nymphs. J. Protozool. 17, 374–384 (1970)
Cossins, A. R.: Thelohania contejeani Henneguy, microsporidian parasite of Austropotamobius pallipes Lereboullet—an histological and ultrastructural study. In: Freshwater crayfish. S. Abrahamsson, ed. Lund: Studentlitteratur 1973
Gurley, R. R.: The Myxosporidia, or psorosperms of fishes, and the epidemics produced by them. Bull. U.S. Fish. Comm. 1892, 190–205 (1893)
Hazard, E. I., Weiser, J.: Spores of Thelohania in adult female Anopheles: development and transovarial transmission, and redescriptions of T. legeri and T. obesa Kudo. J. Protozool. 15, 817–823 (1968)
Marchesi, V. T., Palade, G. E.: The localization of Mg-Na-K activated adenosine triphosphatase on red cell host membranes. J. Cell Biol. 35, 385 (1967)
Maurand, J., Fize, A., Fenwick, B., Michel, R.: Étude au microscope électronique de Nosema infirmum Kudo 1921, microsporidie parasite d'un copépode cyclopoïde; création du genre nouveau Tuzetia à propos de cette espèce. Protistologica 7, 221–225 (1971)
Maurand, J., Manier, J.-F.: Une microsporidie nouvelle pour les larves de Simulies. Protistologica 3, 445–449 (1968)
Maurand, J., Vey, A.: Étude histopathologique et ultrastructurale de Thelohania contejeani (Microsporida, Nosematidae) parasite de l'écrevisse Austropotamobius pallipes Lereboullet. Ann. Parasit. (Paris) 48, 411–421 (1973)
Neaves, W. B.: Permeability of Sertoli cell tight junctions to lanthanum after ligation of ductus deferens and ductuli efferentes. J. Cell Biol. 59 559 (1973)
Overstreet, R. M.: Parasites of some penaeid shrimps with emphasis on reared hosts. Aquaculture 2, 105–140 (1973)
Pearse, A. G. E.: Histochemistry-theoretical and applied, vol. 1, 3rd ed. Baltimore: The Williams & Wilkins Co. 1968
Pixell-Goodrich, H. L. M.: The spore of Thelohania. Arch. Zool. exp. gén. N.&R. 59, 17–19 (1920)
Sprague, V.: Some protozoan parasites and hyperparasites in marine decapod Crustacea. In: A symposium on diseases of fishes and shellfishes, S. F. Snieszko, ed. Amer. Fish. Soc. Spec. Publ. No. 5 (1970a)
Sprague, V.: Taxonomy of microsporidia. J. Parasitol. 56, sec. 2, part 1, 327 (1970b)
Szollosi, D.: Development of Pleistophora sp. (microsporidian) in eggs of the polychaete Armandia brevis. J. Inv. Pathol. 18, 1–15 (1971)
Thélohan, P.: Recherches sur les Myxosporidies. Bull. Soc. France Belg. 26, 103–394 (1894)
Vávra, J.: Spore projections in Microsporidia. Acta protozool. 1, 153–155 (1963)
Vávra, J.: Étude au microscope électronique de la morphologie et du développement de quelques Microsporidies. C. R. Acad. Sci. (Paris) 261, 3467–3470 (1965)
Vávra, J.: Ultrastructural features of Caudospora simulii Weiser (Protozoa, Microsporidia). Folia Parasitologica (Praha) 15, 1–9 (1968)
Vernick, S. H., Sprague, V.: In vitro muscle lysis accompanying treatment with extract of crab muscle infected with Nosema sp. J. Parasitol. 56, sec. 2. part 1, 352–353 (1970)
Vey, A., Vago, C.: Protozoan and fungal diseases of Austropotamobius pallipes Lereboullet in France. In: Freshwater crayfish, S. Abrahamsson, ed. Lund: Studentlitteratur 1973
Wachstein, M., Meisel, E.: Histochemistry of hepatic phosphatases at a physiological pH. Amer. J. clin. Path. 27, 13 (1957)
Author information
Authors and Affiliations
Additional information
This study was conducted in cooperation with the U.S. Department of Commerce, NOAA, National Marine Fisheries Service, under PL 88-309, Project No. 2-174-R and NOAA, Office of Sea Grant, under Grant No. 04-3-158-53. Additional support was from National Science Foundation Research Grant GA-36198. The U.S. Government is authorized to produce and distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon.
Rights and permissions
About this article
Cite this article
Overstreet, R.M., Weidner, E. Differentiation of microsporidian spore-tails in Inodosporus spraguei Gen. et Sp. N.. Z. F. Parasitenkunde 44, 169–186 (1974). https://doi.org/10.1007/BF00328760
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00328760