Abstract
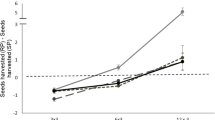
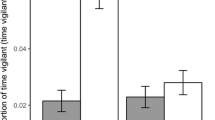
Predation plays an important role in ecological communities by affecting prey behavior such as foraging and by physical removal of individual prey. In regard to foraging, animals such as desert rodents often balance conflicting demands for food and safety. This has been studied in the field by indirectly manipulating predatory risk through the alteration of cues associated with increased risk such as cover or illumination. It has also been studied by directly manipulating the presence of predators in aviaries. Here, we report on experiments in which we directly manipulated actual predatory risk to desert rodents in the field. We conducted a series of experiments in the field using a trained barn owl (Tyto alba) to investigate how two species of coexisting gerbils (Gerbillus allenbyi and G. pyramidum) respond to various cues of predatory risk in their natural environment. The gerbils responded to risk of predation, in the form of owl flights and owl hunger calls, by reducing their activity in the risky plot relative to the control plot. The strongest response was to owl flights and the weakest to recorded hunger calls of owls. Furthermore, when risk of predation was relatively high, as in the case with barn owl flights, both gerbil species mostly limited their activity to the safer bush microhabitat. The response of the gerbils to risk of predation disappeared very quickly following removal of the treatment, and the gerbils returned to normal levels of activity within the same night. The gerbils did not respond to experimental cues (alarm clock), the presence of the investigators, the presence of a quiet owl, and recorded “white noise”. Using trained barn owls, we were able to effectively manipulate actual risk of predation to gerbils in natural habitats and to quantify how gerbils alter their behavior in order to balance conflicting demands of food and safety. The method allows assessment of aspects of behavior, population interactions, and community characteristics involving predation in natural habitats.
Similar content being viewed by others
References
Abrahams MV, Dill LM (1989) A determination of the energetic equivalence of the risk of predation. Ecology 70:999–1007
Abramsky Z (1984) Population biology of Gerbillus allenbyi Mammalia 48:197–206
Abramsky Z (1988) The role of habitat and productivity in structuring desert rodent communities. Oikos 52:107–114
Abramsky Z, Sellah C (1982) competition and the role of habitat selection in Gerbillus allenbyi and Meriones tristrami: a removal experiment. Ecology 63:1242–1247
Abramsky Z, Rosenzweig ML (1984) Tilman's predicted productivity-diversity relationship shown by desert rodents. Nature 309:150–151
Abramsky Z, Brand S, Rosenzweig ML (1985) Geographical ecology of gerbilline rodents in sand dune habitats of Israel. J Biogeogr 12:363–372
Abramsky Z, Pinshow B (1989) Changes in foraging effort in two gerbil species correlate with habitat type and intra-and inter-specific activity. Oikos 56:43–53
Abramsky Z, Rosenzweig ML, Pinshow B, Brown JS, Kotler BP, Mitchell WA (1990) Habitat selection: an experimental field test with two gerbil species. Ecology 71:2358–2369
Abramsky Z, Rosenzweig ML, Pinshow B (1991) The shape of a gerbil isocline. Ecology 72:329–340
Abramsky Z, Ovadia O, Rosenzweig ML (1994) The shape of a Gerbillus pyramidum (Rodentia: Gerbillinae) isocline: an experimental field study. Oikos 69:318–326
Anderson PK (1986) Foraging range in mice and voles: the role of risk. Can J Zool 64:2645–2653
Bar Y, Abramsky Z, Gutterman Y (1984) Diet of gerbilline rodents in the Israeli desert. J Arid Environ 7:371–376
Brown JS (1986) Coexistence on a resource whose abundance varies: a test with desert rodents. Ph.D dissertation, University of Arizona, tucson
Brown JS (1988) Patch use as an indicator of habitat preference, predation risk and competition. Behav Ecol Sociobiol 22:37–47
Brown JS (1989) Desert rodent community structure: a test of four mechanisms of coexistence. Ecol Monogr 59:1–22
Brown JS, Alkon PU (1990) Testing values of crested porcupine habitats by experimental food patches. Oecologia 83:512–518
Brown JS, Kotler BP, Smith RJ, Wirtz WO (1988) The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia 76:408–415
Brown JS, Kotler BP, Mitchell WA (in press) Patch use as the basis for the structure of a Negev desert granivore community. Ecology
Caraco T, Martindale S, Pulliam HR (1980) Avian flocking in the presence of a predator. Nature 285:400–401
Cassini MH (1991) Foraging under predation risk in the wild guinea pig Cavia aperea. Oikos 62:20–24
Cerri RD, Fraser DF (1983) Predation and risk in foraging minnows: balancing conflicting demands. Am Nat 121:552–561
Clarke JA (1983) Moonlight's influence on predator/prey interactions between short-eared owls (Asio flammeus) and deer mice (Peromyscus maniculatus). Behav Ecol Sociobiol 13:205–209
Danin A (1978) Plant species diversity and plant succession in sandy area in the northern Negev. Flora 167:409–422
Dill LM, Fraser AHG (1984) Risk of predation and the feeding behavior of juvenile coho salmon (Oncorhynchus kisutch). Behav Ecol Sociobiol 16:65–71
Doucet GJ, Bider JR (1969) Activity of Microtus pennsylvanicus related to moon phase and moonlight revealed by the sand transect technique. Can J Zool 47:1183–1186
Edwards J (1983) Diet shifts in moose due to predator avoidance. Oecologia 60:185–189
Fretwell SD (1972) Populations in a seasonal environment. Princeton University Press, Princeton
Gilliam JF, Fraser DF (1987) Habitat selection under predation hazard: test of a model with foraging minnows. Ecology 68:1856–1862
Holbrook SJ, Schmitt RJ (1988) The combined effects of predation risk and food reward on patch selection. Ecology 69:125–134
Kotler BP (1984a) Harvesting rates and predatory risk in desert rodents: a comparison of two communities on different continents. J Mammal 65:91–96
Kotler BP (1984b) Risk of predation and the structure of desert rodents communities. Ecology 65:689–701
Kotler BP (1985b) Microhabitat utilization in desert rodents: a comparison of two methods of measurement. J Mammal 66:374–378
Kotler BP, Brown JS (1988) Environmental heterogeneity and the coexistence of desert rodents. Annu Rev Ecol Syst 19:281–308
Kotler BP, Holt RD (1989) Predation and competition: the interaction of two types of species interactions. Oikos 54:256–260
Kotler BP, Brown JS, Smith RJ, Wirtz WO (1988) The effects of morphology and body size on rates of owl predation on desert rodents. Oikos 53:145–152
Kotler BP, Brown JS, Hasson O (1991) The specter of predation: factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72:2249–2260
Kotler BP, Blaustein L, Brown JS (1992) Predator facilitation: the combined effect of snakes and owls on the foraging behavior of gerbils. Ann Zool Fenn 29:199–206
Kotler BP, Blaustein L, Dednam H (1993a) The specter of predation: the effect of vipers on the foraging behavior of two species of gerbils. Isr J Zool 39:11–22
Kotler BP, Brown JS, Slotow R, Goodfriend W, Strauss M (1993b) the influence of snakes on the foraging behavior of gerbils. Oikos 67:309–316
Kotler BP, Brown JS, Subach A (1993c) Mechanisms of species coexistence of optimal foragers: temporal partitioning by two species of sand dune gerbils. Oikos 67:548–556
Lima SL (1985) Maximizing feeding efficiency and minimizing time exposed to predators: a trade-off in the black-capped chickadee. Oecologia 66:60–67
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lima SL, Valone TJ, Caraco T (1985) Foraging-efficiency-predation-risk trade-off in the grey squirrel. Anim Behav 33:155–165
Lockard RD, Owings DH (1974) Moon-related surface activity of bannertail (Dipodomys spectabilis and Dipodomys nitratoides) kangaroo rats. Anim Behav 22:262–273
Longland WS, Price MV (1991) Direct observations of owls and heteromyid rodents: can predation risk explain microhabitat use? Ecology 72:2261–2273
Magnhagen C (1991) Predation risk as a cost of reproduction. Trends Ecol Evol 6:183–186
Milinski M, Heller R (1978) Influence of a predator on the optimal foraging behavior of sticklebacks (Gasterosteus aculeatus L.). Nature 275:642–644
Mitchell WA, Abramsky Z, Kotler BP, Pinshow B, Brown JS (1990) The effect of competition on foraging activity in desert rodents: theory and experiments. Ecology 71:844–854
Nonacs P, Dill LM (1990) Mortality risk vs. food quality tradeoffs in a common currency: ant patch preferences. Ecology 71:1886–1892
Ohman MD, Forst BW, Cohen EB (1983) Reverse dial vertical migration: an escape from invertebrate predators. Science 220:1404–1407
Price MV, Waser NM, Bass TA (1984) Effects of moonlight on microhabitat use by desert rodents. J Mammal 65:353–356
Rosenzweig ML, Abramsky Z (1985) Detecting density-dependent habitat selection. Am Nat 126:405–417
Rosenzweig ML, Abramsky Z, (1986) Centrifugal community organization. Oikos 46:339–348
Rosenzweig ML, Abramsky Z, Brand S (1984) Estimating species interactions in heterogeneous environments. Oikos 43:329–340
Sih A (1980) Optimal behavior: can foragers balance two conflicting demands?. Science 210:1041–1043
Sih A (1982) Foraging strategies and the avoidance of predation by an aquatic insect, Notonecta hoffmanni. Ecology 63:786–796
Stamp NE, Bowers MD (1991) Indirect effect on survivorship of caterpillars due to presence of invertebrate predators. Oecologia 88:325–330
Thompson SD (1982) Structure and species composition of desert Heteromyid rodent species assemblages: effects of a simple habitat manipulation. Ecology 63:1313–1321
Werner EE, Gilliam JF, Hall DL, Mittelbach GG (1983) An experimental test of the effects of predation risk on habitat use in fish. Ecology 64:1540–1548
Ziv Y, Abramsky Z, Kotler BP, Subach A (1993) Interference competition and temporal and habitat partitioning in two gerbil species. Oikos 66:237–246
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abramsky, Z., Strauss, E., Subach, A. et al. The effect of barn owls (Tyto alba) on the activity and microhabitat selection of Gerbillus allenbyi and G. pyramidum . Oecologia 105, 313–319 (1996). https://doi.org/10.1007/BF00328733
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00328733