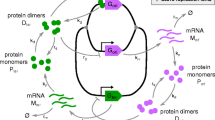
Abstract
Regulation of gene expression in prokaryotic cells usually takes place at the level of transcription initiation. Different forms of RNA polymerase recognizing specific promoters are engaged in the control of many prokaryotic regulons. This also seems to be the case for some Escherichia coli genes that are induced at low growth rates and by nutrient starvation. Their gene products are synthesized at levels inversely proportional to growth rate, and this mode of regulation has been termed gearbox gene expression. This kind of growth-rate modulation is exerted by specific transcriptional initiation signals, the gearbox promoters, and some of them depend on a putative new σ factor (RpoS). Gearbox promoters drive expression of morphogenetic and cell division genes at constant levels per cell and cycle to meet the demands of cell division and septum formation. A mechanism is proposed that could sense the growth rate of the cell to alter gene expression by the action of specific σ factors
Similar content being viewed by others
References
Aldea, M., Garrido, T., Hernández-Chico, C., Vicente, M. & Kushner, S.R. 1989 Induction of a growth-phase-dependent promoter triggers transcription of bolA, an E. coli morphogene. EMBO Journal 8, 3923–3931.
Aldea, M., Garrido, T., Pla, J. & Vicente, M. 1990 Division genes in Escherichia coli are expressed coordinately to cell septum requirements by gearbox promoters. EMBO Journal 9, 3787–3794.
Aldea, M., Hernández-Chico, C., De LaCampa, A.G., Kushner, S.R. & Vicente, M. 1988 Identification, cloning, and expression of bolA, an ftsZ-dependent morphogene of Escherichia coli. Journal of Bacteriology 170, 5169–5176.
Bohannon, D., Connell, N., Keener, J., Tormo, A., Espinosa-Urgel, M., Zambrano, M. & Kolter, R. 1991 Stationary-phase inducible “gearbox” promoters: differential effects of katF mutations and role of 419-1. Journal of Bacteriology 73, 4482–4492.
Connell, N., Han, Z., Moreno, F. & Kolter, R. 1987 An E. coli promoter induced by the cessation of growth. Molecular Microbiology 1, 195–201.
Dassa, E. & Bouquet, P.L. 1985 Identification of the gene appA for the acid phosphatase (pH optimum 2.5) of Escherichia coli. Molecular and General Genetics 200, 68–73.
DelCastillo, I., Gomez, J.M. & Moreno, F. 1990 mrpA, an Escherichia coli gene that reduces growth-phase-dependent synthesis of microcins B17 and C7 and blocks osmoinduction of proU when cloned on a high-copy-number plasmid. Journal of Bacteriology 172, 437–445.
Díaz-Guerra, L., Moreno, F. & San Millán, J.L., 1989 appR gene product activates transcription of microcin C17 plasmid genes. Journal of Bacteriology 171, 2906–2908.
Dopazo, A., Palacios, P., Sánchez, M., Pla, J. & Vicente, M. 1992 An amino-proximal domain required for the localization of FtsQ in the cytoplasmic membrane, and for its biological function in Escherichia coli. Molecular Microbiology 6, 715–722.
Gaal, T. & Gourse, R.L. 1990 Guanosine 3′-diphosphate 5′-disphosphate is not required for growth rate-dependent control of rRNA synthesis in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 87, 5533–5537.
Genilloud, O., Moreno, F. & Kolter, R. 1989 DNA sequence, products and transcriptional pattern of the genes involved in production of the DNA replication inhibitor microcin B17. Journal of Bacteriology 171, 1126–1135.
Gourse, R.L., DeBoer, H.A. & Nomura, M. 1986 DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell 44, 197–205.
Hengge-Aronis, R., Klein, W., Lange, R., Rimmele, M. & Boos, W. 1991 Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. Journal of Bacteriology 173, 7918–7924.
Hernández-Chico, C., San Millán, J.L., Kolter, R. & Moreno, F. 1986 Growth phase and OmpR regulation of transcription of the microcin B17 genes. Journal of Bacteriology 167, 1058–1065.
Jensen, K.F. & Pedersen, S. 1990 Metabolic growth rate control in Escherichia coli may be a consequence of subsaturation of the macromolecular biosynthetic apparatus with substrates and catalytic components. Microbiological Reviews 54, 89–100.
Jung, J.U., Gutierrez, C., Martin, F., Ardourel, M. & Villarejo, M. 1990 Transcription of osmB, a gene encoding an Escherichia coli lipoprotein, is regulated by dual signals. Journal of Biological Chemistry 265, 10574–10581.
Kaasen, I., Falkenberg, P., Styrvold, O.B. & Strom, A.R. 1992 Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: evidence that transcription is activated by KatF (AppR). Journal of Bacteriology 174, 889–898.
Kolter, R. 1992 Life and death in stationary phase. ASM News 58, 75–79.
Laere, A.V. 1989 Trehalose, reserve and/or stress metabolite? FEMS Microbiological Reviews 63, 201–210.
Lange, R. & Hengge-Aronis, R. 1991a Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor 419-2. Journal of Bacteriology 173, 4474–4481.
Lange, R. & Hengge-Aronis, R. 1991b Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Molecular Microbiology 5, 49–59.
Lindahl, L. & Zengel, J.M. 1986 Ribosomal genes in Escherichia coli. Annual Review of Genetics 20, 297–236.
Loewen, P.C. 1984 Isolation of a catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. Journal of Bacteriology 157, 622–626.
Matin, A. 1991 The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Molecular Microbiology 5, 3–10.
Matin, A., Auger, E.A., Blum, P.H. & Schultz, J.E. 1989 Genetic basis of starvation survival in non differentiating bacteria. Annual Review of Microbiology 43, 293–316.
McCann, M.P., Kidwell, J.P. & Matin, A. 1991 The putative s factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. Journal of Bacteriology 173, 4188–4194.
McClure, W.R. 1980 Rate-limiting steps in RNA chain initiation. Proceedings of the National Academy of Sciences of the United States of America 77, 5634–5638.
Mulvey, M.R. & Loewen, P.C. 1989 Nucleotide sequence of katF of Escherichia coli suggests KatF protein is a novel s transcription factor. Nucleic Acids Research 17, 9979–9991.
Mulvey, M.R., Sorby, P.A., Triggs-Raine, B.L. & Loewen, P.C. 1988 Cloning and physical characterization of katE and katF required for catalase HPII expression in Escherichia coli. Gene 73, 337–345.
Mulvey, M.R., Switala, J., Borys, A. & Loewen, P.C. 1990 Regulation of transcription of katE and katF in Escherichia coli. Journal of Bacteriology 172, 6713–6720.
Nomura, M., Gourse, R. & Baughman, G. 1984 Regulation of the synthesis of ribosomes and ribosomal components. Annual Review of Biochemistry 53, 75–117.
Pla, J., Dopazo, A. & Vicente, M. 1990 The native form of FtsA, a septal protein of Escherichia coli, is located in the cytoplasmic membrane. Journal of Bacteriology 172, 5097–6102.
Repoila, F. & Gutiérrez, C. 1991 Osmotic induction of the periplasmic trehalase in Escherichia coli K12: characterization of the treA promoter. Molecular Microbiology 5, 747–755.
Romeo, T. & Preiss, J. 1989 Genetic regulation of glycogen biosynthesis in Escherichia coli: in vitro effects of cyclic AMP and guanosine 5′-diphosphate-3′-disphosphate and analysis of in vitro transcripts. Journal of Bacteriology 171, 2773–2782.
Sak, B.D., Eisenstark, A. & Touati, D. 1989 Exonuclease III and the catalase hydroperoxidase II in Escherichia coli are both regulated by the katF gene product. Proceedings of the National Academy of Sciences of the United States of America 86, 3271–3275.
Saporito, S.M., Smith-White, B.J. & Cunningham, R.P. 1988 Nucleotide sequence of the xthA gene of Escherichia coli K12. Journal of Bacteriology 170, 4542–4547.
Schellhorn, H.E. & Hassan, H.M. 1988 Transcriptional regulation of katE in Escherichia coli K-12. Journal of Bacteriology 170, 4286–4292.
Tormo, A., Almiron, M. & Kolter, R. 1990 surA, an Escherichia coli gene essential for survival in stationary phase. Journal of Bacteriology 172, 4339–4347.
Touati, E., Dassa, E. & Bouquet, P.L. 1986 Pleiotropic mutations in appR reduce pH 2.5 acid phosphatase expression and restore succinate utilisation in CRP-deficient strains of E. coli. Molecular and General Genetics 202, 257–264.
Touati, E., Dassa, E., Bouquet, P.L. & Touati, D. 1991 Are appR and kafF the same Escherichia coli gene encoding a new sigma transcription initiation factor? Research in Microbiology 142, 29–36.
Vicente, M., Kushner, S.R., Garrido, T. & Aldea, M. 1991 The role of the “gearbox” in the transcription of essential genes. Molecular Microbiology 5, 2085–2091.
VonOssowski, I., Mulvey, M.R., Leco, P.A., Borys, A. & Loewen, P.C. 1991 Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. Journal of Bacteriology 173, 514–520.
Ward, J.E. & Lutkenhaus, J. 1985 Overproduction of FtsZ induces minicell formation in E. coli. Cell 42, 941–949.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aldea, M., Garrido, T. & Tormo, A. Gearbox gene expression and growth rate. World Journal of Microbiology and Biotechnology 9, 414–420 (1993). https://doi.org/10.1007/BF00328029
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00328029