Abstract
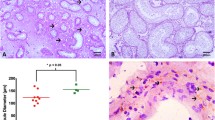
The morphological response of the Sertoli cells to partial or complete withdrawal of testosterone was studied in adult rats following hypophysectomy or administration of ethane dimethanesulphonate (EDS), a toxicant known to destroy selectively the Leydig cells of the testis. To assess the role of germ cells in effecting changes to Sertoli cells following withdrawal of testosterone, germ cell-deficient rats with Sertoli-cell-only testes (SCO) were treated with EDS to remove the source of testosterone. At 6 days after hypophysectomy or 4,6 and 8 days after EDS treatment, stage VII and VIII seminiferous tubules showed degenerating germ cells and numerous basally-located vacuoles approximately 1–15 μm in diameter. Ultrastructural analysis indicated that most of the vacuoles were multiple focal dilations of the intercellular space associated with Sertoli cell junctional complexes. In SCO rats, treatment with EDS resulted in a significant (P<0.05) increase in the formation of many vacuoles particularly in the base but also in the trunk of the Sertoli cells and again electron microscopic analysis showed multiple, localized expansions of the intercellular space associated with Sertoli cell junctional complexes. The appearance of intercellular spaces in SCO testes following androgen withdrawal cannot be attributed to shrinkage of degenerating germ cells since the seminiferous tubules did not contain germ cells. It is concluded that withdrawal of androgen induces early morphological alterations of the Sertoli cell junctional complexes in which the sites of membrane fusions representing tight junctions remain intact whereas the intercellular spaces exhibit major focal dilations. The results are discussed in relation to the fluid secretion by the seminiferous tubules which is regulated by the Sertoli cells.
Similar content being viewed by others
References
Bartlett JMS, Kerr JB, Sharpe RM (1986) The effect of selective destruction and regeneration of rat Leydig cells on the intratesticular distribution of testosterone and morphology of the seminiferous epithelium. J Androl 7:240–253
Bartlett JMS, Kerr JB, Sharpe RM (1988) The selective removal of pachytene spermatocytes using methoxy acetic acid as an approach to the study in vivo of paracrine interactions in the testis. J Androl 9:31–40
Bergh A (1983) Early morphological changes in the abdominal testes in immature unilaterally cryptorchid rats. Int J Androl 6:73–90
Byers S, Graham R (1990) Distribution of sodium potassium AT-Pase in the rat testis and epididymis. Am J Anat 188:31–43
Clermont Y, Morgentaler H (1955) Quantitative study of sperma-togenesis in the hypophysectomized rat. Endocrinology 57:369–382
Creasy DM, Beech LM, Gray TJB, Butler WH (1987) The ultrastructural effects of di-n-pentyl phthalate on the testis of the mature rat. Exp Mol Pathol 46:357–371
de Kretser DM (1987) Local regulation of testicular function. Int Rev Cytol 109:89–111
de Kretser DM, Kerr JB (1988) The cytology of the testis. In: Knobil E, Neill J (eds) The Physiology of Reproduction. Raven Press, New York, pp 837–932
Dym M, Fawcett DW (1970) The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 3:308–326
Fawcett DW (1975) Ultrastructure and function of the Sertoli cell. In: Hamilton DW, Greep RO (eds) Handbook of Physiology, sect 7, vol V. American Physiological Society, Washington, DC, pp 21–55
Fawcett DW (1977) The ultrastructure and functions of the Sertoli cell, In: Greep RO, Koblinsky MA (eds) Frontiers in Reproduction and Fertility Control. MIT Press, Cambridge, pp 302–320
Flickinger CJ (1981) Focal changes in the seminiferous tubules of vasectomized hamsters. J Androl 5:269–277
Ghosh S, Sinha-Hikim AP, Russell LD (1991) Further observations of stage-specific effects seen after short-term hypophysectomy in the rat. Tissue Cell 23:613–630
Ghosh S, Bartke A, Grasso P, Reichert LE, Russell LD (1992) Structural manifestations of the rat Sertoli cell to hypophysectomy: a correlative morphometric and endocrine study. Endocrinology 131:485–497
Gravis CJ, Chen I, Yates RD (1977) Stability of the intra-epithelial component of the blood-testis barrier in epinephrine-induced testicular degeneration in Syrian hamsters. Am J Anat 148: 19–32
Grootegoed JA, Peters MJ, Mulder E, Rommerts FFG, Molen HJ van der (1977) Absence of a nuclear androgen receptor in isolated germ cells of rat testis. Mol Cell Endocrinol 9:159–167
Grove BD, Pfeiffer DC, Allen S, Vogl AW (1990) Immunofluorescence localization of vinculin in ectoplasmic specializations of rat Sertoli cells. Am J Anat 188:44–56
Hagenas L, Ploen L, Ritzen EM, Ekwall H (1977) Blood-testis barrier: maintained function of inter-Sertoli cell junctions in experimental cryptorchidism in the rat, as judged by a simple lanthanum-immersion technique. Andrologia 9:250–254
Hagenas L, Ploen L, Ekwall H (1978) Blood-testis barrier: evidence for intact inter-Sertoli cell junctions after hypophysectomy in the adult rat. J Endocrinol 76:87–91
Hoffer AP (1983) Effects of gossypol on the seminiferous epithelium in the rat: a light and electron microscope study. Biol Reprod 28:1007–1020
Jegou B, Le Gac F, Irby DC, de Kretser DM (1983a) Studies on seminiferous tubule fluid production in the adult rat: effect of hypophysectomy and treatment with FSH, LH and testosterond. Int J Androl 6:249–260
Jegou B, Risbridger GP, de Kretser DM (1983b) Effects of experimental cryptorchidism on testicular function in adult rats. J Androl 4:88–94
Kerr JB, Sharpe RM (1989a) Macrophage activation enhances the human chorionic gonadotrophin-induced disruption of spermatogenesis in the rat. J Endocrinol 121:285–292
Kerr JB, Sharpe RM (1989b) Focal disruption of spermatogenesis in the testis of adult rats after a single administration of human chroionic gonadotrophin. Cell Tissue Res 257:163–169
Kerr JB, Rich KA, de Kretser DM (1979) Effects of experimental cryptorchidism on the ultrastructure and function of the Sertoli cell and peritubular tissue of the rat testis. Biol Reprod 21:823–838
Kerr JB, Donachie K, Rommerts FFG (1985) Selective destruction and regeneration of rat Leydig cells in vivo: a new method for the study of seminiferous tubular-interstitial tissue interaction. Cell Tissue Res 242:145–156
Kerr JB, Millar M, Maddocks S, Sharpe RM (1993) Stage-dependent changes in spermatogenesis and Sertoli cells in relation to the onset of spermatogenic failure following withdrawal of testosterone. Anat Rec 235:547–559
Leblond CP, Clermont Y (1952) Definition of the stage of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci 55:548–573
Maddocks S, Kerr JB, Allenby G, Sharpe RM (1992) Evaluation of the role of germ cells in regulating the route of secretion of immunoactive inhibin from the rat testis. J Endocrinol 132:439–448
Means AR, Fakunding AL, Huckins C, Tindall DJ, Vitale R (1976) Follicle-stimulating hormone, the Sertoli cell, and spermatogenesis. Recent Progr Horm Res 32:477–522
O'Leary PC, Jackson AE, Irby DC, de Kretser DM (1987) Effects of ethane dimethane sulphate (EDS) on seminiferous tubule function in rats. Int J Androl 10:625–634
Rich KA, de Kretser DM (1977) Effects of differing degrees of destruction of the rat seminiferous epithelium on levels of serum follicle-stimulating hormone and androgen binding protein. Endocrinology 101:959–968
Rich KA, Kerr JB, de Kretser DM (1979) Evidence for I eydig cell dysfunction in rats with seminiferous tubule damage. Mol Cell Endocrinol 13:123–135
Ross MH, Dobler J (1975) The Sertoli cell junctional specializations and their relationship to the germinal epithelium as observed after efferent duct ligation. Anat Rec 183:267–292
Russell LD (1983) Normal testicular structure and methods of evaluation under experimental and disruptive conditions. In: Clarkson TW, Nordberg GF, Sager PR (eds) Reproductive and Developmental Toxicity of Metals. Plenum Press, New York, pp 227–252
Russell LD, Clermont Y (1977) Degeneration of germ cells in normal, hypophysectomized and hormone-treated hypophysectomized rats. Anat Rec 187:347–366
Sar M, Lubahn DB, French FS, Wilson EM (1990) Immunohisto-chemical localization of the androgen receptor in rat and human tissues. Endocrinology 127:3180–3186
Schulze C (1984) Sertoli cells and Leydig cells in man. Adv Anat Embryol Cell Biol 88:1–104
Setchell BP (1980) The functional significance of the blood-testis barrier. J Androl 1:3–10
Sharpe RM, Donachie K, Cooper I (1988a) Re-evaluation of the intratesticular level of testosterone required for quantitative maintenance of spermatogenesis in the rat. J Endocrinol 117:19–26
Sharpe RM, Fraser HM, Ratnasooriya WD (1988b) Assessment of the role of Leydig cell products other than testosterone in spermatogenesis and fertility in adult rats. Int J Androl 11:507–523
Sharpe RM, Maddocks S, Kerr JB (1990) Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am J Anat 188:3–20
Sharpe RM, Maddocks S, Millar M, Kerr JB, Saunders PTK, McKinnell C (1992) Testosterone and spermatogenesis. Identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J Androl 13:172–184
Steinberger E (1971) Hormonal control of mammalian spermatogenesis. Physiol Rev 51:1–72
Tindall DJ, Miller A, Means AR (1977) Characterization of androgen receptor in Sertoli cell-enriched testis. Endocrinology 101:13–23
Vogl AW, Soucy LJ (1985) Arrangement and possible function of actin filament bundles in ectoplasmic specializations of ground squirrel Sertoli cells. J Cell Biol 100:814–825
Waites GMH, Gladwell RT (1982) Physiological significance of fluid secretion in the testis and blood-testis barrier. Physiol Rev 62:624–671
Waynforth HB (1990) Experimental and surgical technique in the rat. Academic Press, London
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kerr, J.B., Savage, G.N., Millar, M. et al. Response of the seminiferous epithelium of the rat testis to withdrawal of androgen: evidence for direct effect upon intercellular spaces associated with Sertoli cell junctional complexes. Cell Tissue Res 274, 153–161 (1993). https://doi.org/10.1007/BF00327996
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00327996