Abstract
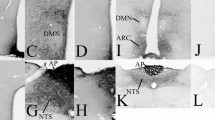
By means of immunocytochemical demonstration of vasoactive intestinal peptide (VIP) an accumulation of cerebrospinal fluid (CSF)-contacting neurons was found in a circumscribed region of the nucleus accumbens/lateral septum of eleven reptilian (chelonian, lacertilian, ophidian, crocodilian) species. Basal processes of these cells contribute to a subependymal plexus whose density displays considerable interspecific variation. VIP-immunoreactive nerve fibers occur also in the lateral septum and the nucleus accumbens where they encompass immunonegative cells in a basket-like pattern. The CSF-contacting neurons are surrounded by columnar ependymocytes frequently arranged in a pseudostratified manner. These specialized arrays of ependymal cells, however, occupy a more extended area than the VIP-immunoreactive CSF-contacting neurons and can be traced from the rostro-ventral pole of the lateral ventricle to the interventricular foramen. These observations suggest the existence of a telencephalic site of CSF-contacting neurons which may be more widespread than hitherto thought and which may participate in a circumventricular system of the lateral ventricle. Previous studies mainly performed with birds indicate that the VIP-immunoreactive CSF-contacting neurons of the nucleus accumbens might form a part of the “encephalic” (extraretinal and extrapineal) photoreceptor. However, further experiments are required to test this supposition since the VIP-immunoreactive neurons of the nucleus accumbens remained unlabeled by antibodies against bovine rodopsin and chicken cone-opsin in all eleven species analysed in this investigation.
Similar content being viewed by others
References
Agduhr E (1922) Über ein zentrales Sinnesorgan (?) bei den Vertebraten. Z Anat Entw Gesch 66:223–360
Benoit J (1938) Action de divers éclairements localisés dans la région orbitaire sur la gonadostimulation chez le canard male impubère. Croissance testiculaire provoquée par l'eclairment direct de la région hypophysaire. C R Soc Biol (Paris) 127:909–914
Farner DS (1975) Photoperiodic controls in the secretion of gonadotropins in birds. Amer Zool 15 [Suppl] 117–135
Follett BK, Davies DT, Magee V (1975) The rate of testicular development in Japanese quail (Coturnix coturnix japonica) following stimulation of the extraretinal photoreceptor. Experientia (Basel) 31:48–49
Foster RG, Follett BK, Lythgoe JN (1985) Rhodopsin-like sensitivity of extra-retinal photoreceptors mediating the photoperiodic response in quail. Nature 313:50–52
Foster RG, Korf HW, Schalken JJ (1987) Immunocytochemical markers revealing retinal and pineal but not hypothalamic photoreceptor systems in quail. Cell Tissue Res 248:161–167
Foster RG, Timmers AM, Schalken JJ, Grip WJ de (1989) A comparison of some photoreceptor characteristics in the pineal and retina: II. The Djungarian hamster (Phodopus sungorus). J Comp Physiol [A] 165:565–572
Foster RG, Alones V, Garcia-Fernandez JM, Grip WJ de (1993) Opsin localization and chromophore retinoids identified within the basal brain of the lizard Anolis carolinensis. J Comp Physiol [A] (in press)
Frisch K v (1911) Beiträge zur Physiologie der Pigmentzellen in der Fischhaut. Pfluegers Arch 138:319–387
Groos G (1982) The comparative physiology of extraocular photoreception. Experientia (Basel) 38:989–1128
Hartwig HG, Oksche A (1982) Neurobiological aspects of extraretinal photoreceptive systems: structure and function. Experientia 38:991–996
Hof PR, Dietl MM, Charnay Y, Martin JL, Bouras C, Palacios JM, Magistretti PJ (1991) Vasoactive intestinal peptide binding sites and fibers in the brain of the pigeon Columba livia: an autoradiographic and immunohistochemical study. J Comp Neurol 305:393–411
Hofer H (1959) Zur Morphologie der circumventrikulären Organe des Zwischenhirns der Säugetiere. Zool Anz 22:202–251
Hofer H (1965) Circumventrikuläre Organe des Zwischenhirns. In: Hofer H, Schultz AH, Starck D (eds) Primatologia, vol II/2/13. Karger, Basel, pp 1–104
Huber GC, Crosby EC (1929) The nuclei and fiber paths of the avian diencephalon with consideration of telencephalic and certain mesencephalic centers and connections. J Comp Neurol 48:1–225
Kavaliers M (1980) Circadian rhythm of extraretinal photosensitivity in hatchling alligators, Alligator mississippiensis. Photochem Photobiol 32:67–70
Kolmer W (1921) Das “Sagittalorgan” der Wirbeltiere. Z Anat Entw Gesch 60:652–717
Korf B, Rollag MD, Korf HW (1989) Ontogenic development of S-antigen and rod-opsin immunoreactions in retinal and pineal photoreceptors of Xenopus laevis in relation to the onset of melatonin-dependent color-change mechanisms. Cell Tissue Res 258:319–329
Korf HW, Fahrenkrug J (1984) Ependymal and neuronal specializations in the lateral ventricle of the Pekin duck, Anas platyrhynchos. Cell Tissue Res 236:217–227
Korf HW, Simon-Oppermann C, Simon E (1982) Afferent connections of physiologically identified neuronal complexes in the paraventricular nucleus of conscious Pekin ducks involved in regulation of salt-and water balance. Cell Tissue Res 226:275–300
Korf HW, Viglietti-Panzica C, Panzica GC (1983) A Golgi study on the cerebrospinal fluid (CSF)-contacting neurons in the paraventricular nucleus of the Pekin duck. Cell Tissue Res 228:149–163
Korf HW, Foster RG, Ekström P, Schalken JJ (1985) Opsin-like immunoreactivity in the retinae and pineal organs of four mammalian species. Cell Tissue Res 242:645–648
Kuenzel WJ, Tienhoven A van (1982) Nomenclature and location of avian hypothalamic nuclei and associated circumventricular organs. J Comp Neurol 206:293–313
Leonhardt H (1980) Ependym und circumventriculäre Organe. In: Oksche A (ed) Neuroglia I. Handbuch der mikroskopischen Anatomie des Menschen, vol IV/10. Springer, Berlin, pp 177–666
Lopez KH, Jones RE, Seufert DW, Rands MS, Dores RM (1992) Catecholaminergic cells and fibers in the brain of the lizard Anolis carolinensis identified by traditional as well as wholemount immunohistochemistry. Cell Tissue Res 270:319–337
Meredith GA, Smeets WJAJ (1987) Immunocytochemical analysis of the dopamine system in the forebrain and midbrain of Raja radiata: evidence for a substantia nigra and ventral tegmental area in cartilaginous fish. J Comp Neurol 265:530–548
Møller M, Glistrup OV, Olsen W (1983) Contrast enhancement of the brownish horseradish peroxidase-activated 3,3′-diaminobenzidine tetrahydrochloride reaction product in black and white photo-micrography by the use of interference filters. J Histochem Cytochem 32:37–42
Müller B, Peichl L, Grip WJ de, Gery I, Korf HW (1989) Opsin-and S-antigen-like immunoreactions in photoreceptors of the tree shrew retina. Invest Ophthalmol Vis Sci 30:530–535
Oksche A (1976) The neuroanatomical basis of comparative neuroendocrinology. Gen Comp Endocrinol 29:225–239
Oliver J, Baylé JD (1982) Brain photoreceptors for the photo-induced testicular response in birds. Experientia 38:1021–1029
Panzica GC, Korf HW, Ramieri G, Viglietti-Panzica C (1986) Golgi-type and immunocytochemical studies on the intrinsic organization of the periventricular layer of the avian paraventricular nucleus. Cell Tissue Res 243:317–322
Rodríguez EM (1976) The cerebrospinal fluid as a pathway in neuroendocrine integration. J Endocrinol 71:407–443
Rodríguez EM, Peña P, Rodríguez S, Aguado LI (1982) Evidence for the participation of the CSF and periventricular structures in certain neuroendocrine mechanisms. Front Horm Res 9:142–158
Romeis B (1968) Mikroskopische Technik. Oldenbourg, München
Rommel E (1992) Neuronale und vasculäre Spezialisierungen im Nucleus accumbens verschiedener Vertebraten. Thesis, Faculty of Medicine, Justus Liebig University, Giessen
Scharrer E (1964) Photo-neuro-endocrine systems: general concepts. Ann N Y Acad Sci 117:13–22
Silver R, Witkovsky P, Horvath P, Alones V, Barnstable CJ, Lehmann MN (1988) Coexpression of opsin-and VIP-like immunoreactivity in CSF-contacting neurons in the avian brain. Cell Tissue Res 253:189–198
Sims KB, Hoffman DL, Said SI, Zimmerman EA (1980) Vasoactive intestinal polypeptide (VIP) in mouse and rat brain: an immunocytochemical study. Brain Res 186:165–183
Sternberger LA, Hardy PH, Cucults JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunohistochemistry. Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-anti horseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Tienhoven A van, Juhász LP (1962) The chicken telencephalon, diencephalon and mesencephalon in stereotactic coordinates. J Comp Neurol 118:185–198
Tretjakoff D (1913) Die zentralen Sinnesorgane bei Petromyzon. Arch Mikroskop Anat 83:68–117
Underwood H, Groos G (1982) Vertebrate circadian rhythms: retinal and extraretinal photoreception. Experientia 38:1013–1021
Veen T van, Hartwig HG, Müller K (1976) Light-dependent motor activity and photonegative behavior in the eel (Anguilla anguilla L). Evidence for extraretinal and extrapineal photoreception. J Comp Physiol 111:209–219
Vigh B (1971) Das Paraventrikularorgan und das zirkumventrikuläre System. Studia Biologica Hungrica, vol. X. Acad Kiadó, Budapest
Vigh B, Vigh-Teichmann I (1973) Comparative ultrastructure of CSF-contacting neurons. Int Rev Cytol 35:189–251
Vigh-Teichmann I, Vigh B (1974) The infudibular cerebrospinal fluid-contacting neurons. Adv Anat Embryol Cell Biol 50:1–91
Vigh-Teichmann I, Vigh B (1983) The system of cerebrospinal fluid-contacting neurons. Arch Histol Jpn 46:427–468
Yamada S, Mikami S, Yanaihara N (1982) Immunohistochemical localization of vasoactive intestinal polypeptide (VIP)-containing neurons in the hypothalamus of the Japanese quail, Coturnix coturnix. Cell Tissue Res 226:13–26
Yokoyama K, Oksche A, Darden TR, Farner DS (1978) The sites of encephalic photoreception in photoperiodic induction of the growth of the testes in the White-crowned sparrow, Zonotrichia leucophrys gambelit. Cell Tissue Res 189:441–467
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirunagi, K., Rommel, E., Oksche, A. et al. Vasoactive intestinal peptide-immunoreactive cerebrospinal fluid-contacting neurons in the reptilian lateral septum nucleus accumbens. Cell Tissue Res 274, 79–90 (1993). https://doi.org/10.1007/BF00327988
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00327988
Key words
- Cereborospinal fluid-contacting neurons
- Vasoactive intestinal peptide (VIP)
- Immunocytochemistry
- Nucleus accumbens/lateral septum
- Photoreceptors, extraocular
- Rod-opsin
- Cone-opsin
- Clemmys leprosa (Chelonia)
- Varanus monitor, Lacerta sicula (Lacertilia)
- Python reticulatus (Serpentes)
- Crocodylus niloticus (Crocodilia)