Summary
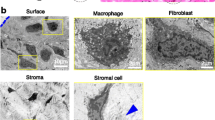
Ontogenetic development of the synovial A cells in fetal rat knee joints was investigated by immunohistochemistry, immuno-electron microscopy, cultivation, and autoradiography. At day 17 of gestation, immature macrophages were first seen in the articular interzone, and thereafter they differentiated into macrophages (synovial A cells), which were found in the synovial intima. The degree of reactivity of macrophages with five monoclonal antibodies increased in the developing synovial membranes of fetal rats as shown by immunohistochemistry. Similar findings were obtained in organ cultures of fetal knee joints. A marked difference of proliferative potential was found between A and B cells during ontogeny. A cells after birth did not incorporate 3H-thymidine in contrast to B cells. Before birth, B cells had a labelling index which was at least five times larger than that of A cells. The results of this study indicate that the synovial A cells are derived from both monocytes and fetal macrophages circulating in peripheral blood and that they differ from the synovial B cells in morphology, differentiation, and proliferative potential.
Similar content being viewed by others
References
Athanasou NA, Quinn J, Heryet A, Puddle B, Woods CG, McGee JO'D (1988) The immunohistology of synovial lining cells in normal and inflamed synovium. J Pathol 155:133–142
Bodel PTN, Nichols BA, Bainton DF (1977) Appearance of peroxidase reactivity within the rough endoplasmic reticulum of blood monocytes after surface adherence. J Exp Med 145:264–274
Burmester GR, Dimitriu-Bona A, Water SJ, Winchester RJ (1983) Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol 17:69–82
Edwards JCW, Wiloughby DA (1982) Demonstration of bone marrow derived cells in synovial lining by means of giant intracellular granules as genetic markers. Ann Rheum Dis 41:177–182
Graabæk PM (1984) Characteristics of the two types of synoviocytes in rat synovial membrane. An ultrastructural study. Lab Invest 6:690–702
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney. Ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14:291–302
Ijima H, Suda T Miura Y (1982) Predominance of macrophagecolony formation in human cord blood. Exp Hematol 10:234–240
Isobe S, Nakane PK, Brown WR (1977) Studies on translocation of immunoglobulins across intestinal epithelium. 1. Improvements to study the peroxidase-labeled antibody method for application to study of human intestinal mucosa. Acta Histochem Cytochem 10:167–171
Kelemen E, Janossa M (1980) Macrophages are the first differentiated blood cells formed in human embryonic liver. Exp Hematol 8:996–1000
Klareskog L, Forsum U, Wigzell H (1982) Murine synovial intima contains I-A-, I-E/C-positive bone-marrow-derived cells. Scand J Immunol 15:509–514
Linck G, Porte A (1978) B-cells of the synovial membrane. Differentiation during development of the synovial cavity in the mouse. Cell Tissue Res 195:251–265
McLean IW, Nakane PK (1974) Periodate-lysine-paraformaldehyde fixative: A new fixative for immunoelectron microscopy. J Histochem Cytochem 22:1077–1083
Mohr W, Beneke G, Moling W (1975) Proliferation of synovial lining cells and fibroblasts. Ann Rheum Dis 34:219–224
Naito M, Takahashi K, Takahashi H, Kojima M (1982) Ontogenetic development of Kupffer cells. In: Wisse E, Knook DL (eds) Sinusoidal liver cells. Elsevier Biomedical Press, Amsterdam, pp 155–164
Naito M, Yamamura F, Takeya M, Takahashi K (1986) Ultrastructural analysis of Kupffer cells progenitors. In: Kirn A, Knook DL, Wisse E (eds) Cells of the hepatic sinusoids, Vol 1. Kupffer Cell Foundation, The Netherlands, pp 13–20
Naito M, Yamamura F, Nishikawa S, Takahashi K (1989) Development, differentiation, and maturation of fetal mouse yolk sac macrophages in cultures. J Leukocyte Biol 46:1–10
Okada Y, Nakanishi I, Kajikawa K (1981) Ultrastructure of the mouse synovial membrane. Development and organization of the extra-cellular matrix. Arthritis Rheum 24:835–843
Palmar DG, Selvendran Y, Allen C, Revell PA, Hogg N (1985) Features of synovial membrane identified with monoclonal antibodies. Clin Exp Immunol 59:529–538
Rhodin JAG (1974) Histology. Oxford University Press, New York London Toronto, pp 140–171
Stofft E, Effendy W (1985) Development and morphology of rat synovial membrane. Acta Anat 121:36–40
Takahashi K, Sakuma H, Naito M, Yaginuma Y, Takahashi H, Asano S, Hojo H, Kojima M (1980) Cytological characters, transformation, and ontogenesis of dermal histiocytes and fibroblasts of rats. Acta Pathol Jpn 30:743–766
Takahashi K, Takahashi H, Naito M, Sato T, Kojima M (1983) Ultrastructural and functional development of macrophages in the dermal tissue of rat fetuses. Cell Tissue Res 232:539–552
Takahashi K, Yamamura F, Naito M (1989) Differentiation, maturation and proliferation of macrophages in the mouse yolk sac. A light microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukocyte Biol 45:87–96
Takeya M, Hsiao L, Takahashi K (1987) A new monoclonal antibody, TRPM-3, binds specifically to certain rat macrophage populations. Immunohistochemical and immunoelectron microscopic analysis. J Leukocyte Biol 41:187–195
Takeya M, Hsiao L, Shimokawa Y, Takahashi K (1989) Heterogeneity of rat macrophages recognized by monoclonal antibodies: An immunohistochemical and immunoelectron microscopic study. J Histochem Cytochem 37:635–641
van Furth R (1980) Cells of the mononuclear phagocyte system: nomenclature in terms of sites and conditions. In: van Furth R (ed) Mononuclear phagocytes: Functional aspects, Pt 1. Martinus Nijhoff Publishers, Hague, pp 1–30
Wacker H-H, Radzun HJ, Parwaresch MR (1985) Ki-M2R, a new specific monoclonal antibody, discriminates tissue macrophages from reticulum cells and monocytes in vivo and in vitro. J Leukocyte Biol 38:509–520
Williams AF, Galfre G, Milstein C (1977) Analysis of cell surface by xenogeneic myeloma-hybrid antibodies. Differentiation antigens of rat lymphocytes. Cell 12:663–673
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Izumi, S., Takeya, M., Takagi, K. et al. Ontogenetic development of synovial A cells in fetal and neonatal rat knee joints. Cell Tissue Res 262, 1–8 (1990). https://doi.org/10.1007/BF00327740
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00327740