Summary
A 6.4 Kb HindIII fragment of Bacillus stearothermophilus DY-5 DNA cloned in Escherichia coli using pBR322 as a vector was shown to direct the synthesis of a thermophilic α-amylase. In attempts to reduce the size of the insert, the α-amylase gene was shown to be contained in a 3.1 Kb HindIII-BamHI fragment of the donor strain DNA.
The α-amylase gene was stably maintained and expressed efficiently in E. coli. The enzymic properties of α-amylase produced in E. coli closely resembled those of the donor strain α-amylase and the temperature range for the maximal activity was from 65° C to 80° C. Nearly 100% of the activity remained after heating at 80° C for 15 min.
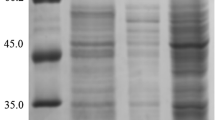
The α-amylase was shown to be accumulated in the periplasmic space. It was purified to a nearly homogenous protein with a molecular weight of 61,000, which was very similar in size to that produced by B. stearothermophilus DY-5.
Similar content being viewed by others
References
Birdsell DC, Cota-Robles EH (1967) Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol 93:427–437
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl Acid Res 7:1513–1523
Brammar WJ, Muir S, McMorris A (1980) Molecular cloning of the gene for the β-lactamase of Bacillus licheniformis and its expression in Escherichia coli. Mol Gen Genet 178:217–224
Cornelis P, Digneffe C, Willemot K (1982) Cloning and expression of a Bacillus coagulans amylase gene in Escherichia coli. Mol Gen Genet 186:507–511
Fuwa H (1954) A new method for microdetermination of amylase activity by the use of amylose as the substrate. J Biochem 41:583–603
Inouye M, Halegoua S (1980) Secretion and membrane localization of proteins in Escherichia coli. Crit Rev Biochem 339–371
Kemp DJ, Cowman AF (1981) Direct immunoassay for detecting Escherichia coli colonies that contain polypeptides encoded by cloned DNA segments. Proc Natl Acad Sci USA 78:4520–4524
Laemmli UK (1970) Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227:680–685
Lederberg EM, Cohen SN (1974) Transformation of Salmonella typhimurium by plasmid DNA. J Bacteriol 119:1072–1074
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Manning GB, Campbell LL (1961) Thermostable α-amylase of Bacillus stearothermophilus I. Crystallization and some general properties. J Biol Chem 236:2952–2957
Ogasahara K, Imanishi A, Isemura T (1970) Studies on thermophilic α-amylase from Bacillus stearothermophilus I. Some general and physico-chemical properties of thermophilic α-amylase. J Biochem 67:65–75
Palva I (1982) Molecular cloning of α-amylase gene from Bacillus amyloliquefaciens and its expression in B. subtilis. Gene 19:81–87
Pfueller SJ, Elliott WH (1969) The extracellular α-amylase of Bacillus stearothermophilus. J Biol Chem 244:48–54
Priest F (1977) Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev 41:711–753
Saito H, Miura K (1963) Preparation of transforming DNA by phenol treatment. Biochim Biophys Acta 72:619–629
Sato T, Yura T (1981) Regulatory mutations conferring constitutive synthesis of major outer membrane proteins (OmpC and OmpF) in Escherichia coli. J Bacteriol 145:88–96
Sharp PA, Sugden B, Sambrook J (1973) Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose-ethidium bromide electrophoresis. Biochemistry 12:3055–3063
Tanaka T, Weisblum B (1975) Construction of a colicin E1-R factor composite plasmid in vitro: Means for amplification of deoxyribonucleic acid. J Bacteriol 121:354–362
Yamada H, Tsukagoshi N, Udaka S (1981) Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol 148:322–332
Yang M, Galizzi A, Henner D (1983) Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucl Acad Res 11:237–249
Author information
Authors and Affiliations
Additional information
Communicated by W. Arber
Rights and permissions
About this article
Cite this article
Tsukagoshi, N., Ihara, H., Yamagata, H. et al. Cloning and expression of a thermophilic α-amylase gene from Bacillus stearothermophilus in Escherichia coli . Molec. Gen. Genet. 193, 58–63 (1984). https://doi.org/10.1007/BF00327414
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00327414