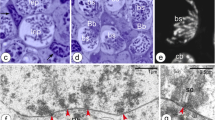
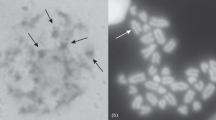
Abstract
Germinal vesicles of oocytes from Pleurodeles waltlii were used for immunization of BALB/c mice to obtain hybridomas secreting monoclonal antibodies. The hybridomas were screened for reactivity of their antibodies against lampbrush chromosomes of oocytes, as revealed by indirect immunostaining. Antibodies labelling the lampbrush chromosomes were also tested on histological sections of oocytes, embryos, and larvae of Pleurodeles. Characterization of the antigens was accomplished through immunoblotting of two-dimensional electrophoretic gels of germinal vesicle proteins. The ten monoclonal antibodies giving a positive reaction were classed into five groups. Group 1, exemplified by antibody A33, recognizes all the lampbrush chromosome transcribing sites (loops). Moreover, it differentially labels the cell nuclei during embryonic and larval development. Group 2, antibody B71, also stains all the loops of the lampbrush chromosomes, but does not react with cell nuclei of embryos and larvae. Group 3, antibody A1, labels specific loops, some of which are heterozygous in the strain of P. waltlii used. These heterozygosities have allowed us to localize and to characterize a chromosomal segment on bivalent IV which is heteromorphic in the two partners of the bivalent. We suggest that this heteromorphism represents a morphological distinction between Z and W heterochromosomes. Moreover, this antibody reacts with only one transcription unit along a loop that contains several units. Group 4, antibody B24, stains the only two structures in the lampbrush chromosomes of P. waltlii that do not have a loop organization, the mass “M” and the spheres. Group 5, antibody A35, reacts with the chromomeres. The antigens corresponding to antibodies A33 and B24 have been identified as proteins, which have apparent molecular weights of 80 and 104 kilodaltons, respectively. They correspond to proteins abundant in the germinal vesicles. All the antibodies described here crossreact with the lampbrush chromosomes of five other species of Urodeles.
Similar content being viewed by others
References
Angelier N, Lacroix JC (1975) Complexes de transcription d'origines nucléolaire et chromosomique d'ovocytes de Pleurodeles waltlii et Pleurodeles poireti (Amphibiens, Urodèles). Chromosoma 51:323–335
Ballantine JEM, Woodland HR, Sturgess EA (1979) Changes in protein synthesis during the development of Xenopus laevis. J Embryol Exp Morphol 51:137–153
Barque JP, Danon F, Peraudeau L, Yeni P, Larsen CJ (1983) Characterization by human autoantibody of a nuclear antigen related to the cell cycle. EMBO J 2:734–749
Barth LG, Barth LJ (1959) Differentiation of cells of the Rana pipiens gastrula in unconditional medium. J Embryol Exp Morphol 7:210–222
Bona M, Scheer U, Bautz EKF (1981) Antibodies to RNA polymerase II (B) inhibit transcription in lampbrush chromosomes after microinjection into living amphibian oocytes. J Mol Biol 151:81–89
Bonnanfant-Jaîs ML, Mentré P (1983) Study of oogenesis in the newt Pleurodeles waltlii M. I. Ultrastructural study of the different stages of oocyte development. J Submicrosc Cytol 15:453–478
Buttin G, Le Guern C, Phalente L, Liu EC, Medrano L, Cazenave PA (1979) Production of hybrid lines secreting monoclonal antiidiotypic antibodies by fusion on membrane filters. In: Melchers F, Potter M, Warned NL (eds) Lymphocyte hybridomas. Springer Verlag, Berlin Heidelberg New York, pp 27–34
Callan HG (1963) The nature of lampbrush chromosomes. Int Rev Cytol 15:1–34
Callan HG (1982) Lampbrush chromosomes. Proc R Soc Lond B 214:417–448
Callan HG, Lloyd L (1956) Visual demonstration of allelic differences within cell nuclei. Nature 178:355–357
Callan HG, Lloyd L (1960) Lampbrush chromosomes of crested newts Triturus cristatus (Laurenti). Phil Trans R Soc Lond B 243:135–219
Callan HG, Lloyd L (1975) Working maps of the lampbrush chromosomes of Amphibia. In: King RC (ed) Handbook of genetics. New York, Plenum Vol 4, pp 57–77
Dequin R, Saumweber H, Sedat JN (1984) Proteins shifting from the cytoplasm into the nuclei during early embryogenesis of Drosophila melanogaster. Dev Biol 104:37–48
Diaz MO, Barsacchi-Pilone G, Mahon KA, Gall JG (1981) Transcripts from both strands of a satellite DNA occur on lampbrush chromosome loops of the newt Notophtalmus. Cell 24:649–659
Dreyer C, Hausen P (1983) Two-dimensional gel analysis of the fate of oocyte nuclear proteins in the development of Xenopus laevis. Dev Biol 109:412–425
Dreyer C, Singer H, Hausen P (1981) Tissue-specific antigens in the germinal vesicle of Xenopus laevis oocytes. Wilhelm Roux's Arch 190:197–207
Dreyer C, Scholz E, Hausen P (1982) The fate of oocyte nuclear proteins during early development in Xenopus laevis. Wilhelm Roux's Arch 191:228–233
Fazekas de St-Groth S, Scheidegger D (1980) Production of monoclonal antibodies: strategy and tactics. J Immunol Methods 35:1–21
Gall JG (1954) Lampbrush chromosomes from oocyte nuclei of the newt. J Morphol 94:283–352
Gall JG, Stephenson EC, Erba HP, Diaz MO, Barsacchi-Pilone G (1981) Histone genes are located at the sphere loci of newt lampbrush chromosomes. Chromosoma 84:159–171
Gall JG, Diaz MO, Stephenson EC, Mahon KA (1983) The transcription unit of lampbrush chromosomes. In: Subtelny S, Kafatos F (eds) Gene structure and regulation in development. Alan R Liss, New York, pp 137–146
Gallien L (1951) Sur la descendance unisexuée d'une femelle de Pleurodeles waltlii (Michah) ayant subi pendant sa phase larvaire l'action gynogène du benzoate d'oestradiol. CR Acad Sci Paris 233:828–830
Gallien L, Durocher M (1957) Table chronologique du développement chez Pleurodeles waltlii (Michah). Bull Biol Fr Belg 91:97–114
Howard GC, Abmayr SM, Shinefeld LA, Sato VL, Elgin SCR (1981) Monoclonal antibodies against a specific non-histone chromosomal protein of Drosophila associated with active genes. J Cell Biol 88:219–225
Hulsebos TJM, Hackstein JHP, Hennig W (1984) Lampbrush loop-specific protein of Drosophila hydei. Proc Natl Acad Sci USA 81:3404–3408
Kabisch R, Bautz EKF (1983) Differential distribution of RNA polymerase B and non-histone chromosomal proteins in polytene chromosomes of Drosophila melanogaster. EMBO J 2:395–402
Kabisch R, Krause J, Bautz EKF (1982) Evolutionary changes in non-histone chromosomal proteins within the Drosophila melanogaster group revealed by monoclonal antibodies. Chromosoma 85:531–538
Karsenti E, Gounon P, Bornens M (1978) Immunocytochemical study of the lampbrush chromosomes: Presence of tubulin and actin. Biol Cell 31:219–224
Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497
Krohne G, Stick R, Kleinschmidt A, Moll R, Franke WW, Hausen P (1982) Immunological localization of a major karyoskeletal protein in nucleoli of oocytes and somatic cells of Xenopus laevis. J Cell Biol 94:749–754
Kuo CH, Gilon H, Blumenthal AB, Sedat JW (1982) A library of monoclonal antibodies to nuclear proteins from Drosophila melanogaster embryos. Exp Cell Res 142:141–154
Lacroix JC (1968a) Etude descriptive des chromosomes en écouvillon dans le genre Pleurodeles (Amphibien, Urodèle). Ann Embryol Morphol 1:179–202
Lacroix JC (1968b) Variations expérimentales ou spontanées de la morphologie et de l'organisation des chromosomes en écouvillon dans le genre Pleurodeles (Amphibien, Urodèle). Ann Embryol Morphol 1:205–248
Lacroix JC (1970) Mise en évidence sur les chromosomes en écouvillon de Pleurodeles poireti Gervais, Amphibien Urodèle, d'une structure liée au sexe identifiant le bivalent sexuel et marquant le chromosome W. CR Acad Sci Paris 271:102–104
Lacroix JC, Loones MT (1971) Fragmentation par les rayons X de l'organisateur d'une différenciation de chromosome en écouvillon (lampbrush) chez Pleurodeles waltlii. Chromosoma 36:112–118
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Littlefield JW (1964) Selection of hybrids from mating fibroblasts in vitro and their presumed recombinants. Science 145:709–710
Macgregor HC (1980) Recent developments in the study of lampbrush chromosomes. Heredity 44:3–35
Macgregor HC, Horner H (1980) Heteromorphism for chromosome I, a requirement for normal development in crested newts. Chromosoma 76:111–122
Makarov VB, Safronov VV (1976) Functional organization of chromomere. III. Analysis of transcriptional units in chromomeres of Triturus cristatus cristatus. Tsitologiya 18:290–295
Miller OL, Beatty BR (1969) Visualization of nucleolar genes. Science 164:955–957
Moreau N, Boucher D (1981) Une méthode rapide d'extraction en masse des noyaux d'ovocytes de Pleurodèle (Amphibien, Urodèle). Biol Cell 42:185–188
Nardi I, Ragghianti M, Mancino G (1972) Characterization of the lampbrush chromosomes of the marbled newt Trituras marmoratus (Latreille, 1800). Chromosoma 37:1–22
O'Farrell PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
Old RW, Callan HG, Gross KW (1977) Localization of histone gene transcripts in newt lampbrush chromosomes by in situ hybridization. J Cell Sci 27:57–79
Peterson JL, McConkey EH (1976) Nonhistone chromosomal proteins from HeLa cells. J Biol Chem 251:548–554
Risau W, Symmons P, Saumweber H, Frasch M (1983) Nonpackaging and packaging proteins of hnRNA in Drosophila melanogaster. Cell 33:529–541
Saumweber H, Symmons P, Kabisch R, Will H, Bonhoeffer F (1980) Monoclonal antibodies against chromosomal proteins of Drosophila melanogaster. Chromosoma 80:253–275
Scheer U, Franke WW, Trendelenburg MF, Spring H (1976) Classification of loops of lampbrush chromosomes according to the arrangement of transcriptional complexes. J Cell Sci 22:503–520
Scheer U, Sommerville J, Bustin M (1979) Injected histone antibodies interfere with transcription of lampbrush chromosomes loops in oocytes of Pleurodeles. J Cell Sci 40:1–20
Schulman M, Wilde CD, Köhler CA (1978) A better cell line for making hybridomas secreting specific antibodies. Nature 276:269–270
Scott SEM, Sommerville J (1974) Localization of nuclear proteins on the chromosomes of newt oocytes. Nature 250:680–682
Sommerville J (1977) Gene activity in the lampbrush chromosomes of amphibian oocytes. Int Rev Biochem 15:79–156
Sommerville J (1981) Immunolocalization and structural organization of nascent RNP. In: Busch H (ed) The cell nucleus, vol VIII Academic Press, New York, pp 1–57
Sommerville J, Crichton C, Malcolm D (1978) Immunofluorescent localization of transcriptional activity on lampbrush chromosomes. Chromosoma 66:99–114
Steedman HF (1957) Polyester wax. A new ribboning embedding medium for histology. Nature 179:1345
Straznicky K, Gaze RM (1972) The development of the tectum in Xenopus laevis: An autoradiographic study. J Embryol Exp Morphol 28:87–115
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Turner BM (1981) Isolation of monoclonal antibodies to chromatin and preliminary characterization of target antigens. Eur J Cell Biol 24:266–274
Vanderbilt JN, Anderson JN (1983) Monoclonal antibodies to tissue-specific chromatin proteins. J Biol Chem 258:7751–7756
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lacroix, J.C., Azzouz, R., Boucher, D. et al. Monoclonal antibodies to lampbrush chromosome antigens of Pleurodeles waltlii . Chromosoma 92, 69–80 (1985). https://doi.org/10.1007/BF00327246
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00327246