Summary
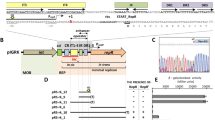
By deletion analysis we have defined a 1.1 kb segment required for driving autonomous replication of the plasmid pC194. The minimal replicon specifies a positive, RepH, and a negative, Inc8A, trans-acting product and their target sites. The RepH product has a M4 of 34.1 kDa, could be overproduced, and binds specifically to the pC194 origin region. By trans complementation studies we have shown that pC194 replication is indirectly controlled at the level of RepH synthesis by a negative product, IncA, that is transcribed within the repH transcription unit in the opposite direction (“antisense RNA”). The antisense RNA regulates the RepH synthesis by a mechanism that seems to involve RNA/RNA interaction in a manner that interferes with translation. In addition, an autoregulatory control might be operative.
Similar content being viewed by others
References
Alonso JC, Trautner TA (1985a) A gene controlling segregation of the Bacillus subtilis plasmid pC194. Mol Gen Genet 198:427–431
Alonso JC, Trautner TA (1985b) Cold sensitivity in the transfer of a plasmid with a deletion hot spot into recombination deficient B. subtilis cells. Mol Gen Genet 198:437–440
Alonso JC, Lüder G, Trautner TA (1986) Requirement for the formation of plasmid-transducing particles of Bacillus subtilis bacteriophage SPP1. EMBO J 5:3723–3728
Alonso JC, Viret J-F, Tailor RH (1987) Plasmid maintenance in Bacillus subtilis recombination-deficient mutants. Mol Gen Genet 208:349–352
Ballester S, Lopez P, Alonso JC, Espinosa M, Lacks SA (1986) Selective advantage of plasmid deletions affecting chloramphenicol acety transferase gene expression. Gene 41:153–163
Biswal N, Kleinschmidt HC, Spatz HC, Trautner TA (1967) Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet 100:39–55
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brendel V, Trifanov EN (1984) A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res 12:4411–4427
Chang S, Cohen SN (1979) High frequency transformation of B. subtilis protoplasts by plasmid DNA Mol Gen Genet 168:111–115
Dagert M, Jones I, Goze A, Romac S, Niaudet B, Ehrlich SD (1984) Replication functions of pC194 are necessary for efficient plasmid transduction by M13 phage. EMBO J 3:81–86
Deichelbohrer I, Alonso JC, Lüder G, Trautner TA (1985) Plasmid transduction by Bacillus subtilis bacteriophage SPP1: Effects of DNA homology between plasmid and bacteriophage. J Bacteriol 162:1238–1243
Diaz R, Ortega S (1984) Initiation of R1 replication in vitro is independent of transcription by host RNA polymerase. Nucleic Acids Res 12:5175–5191
Ehrlich SD (1977) Replication and expression of plasmid from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci USA 74:1680–1682
Gryczan TJ, Contente S, Dubnau D (1978) Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol 134:318–329
Hahn J, Dubnau D (1985) Analysis of plasmid deletional instability in Bacillus subtilis. J Bacteriol 162:1014–1023
Horinouchi S, Weisblum B (1982) Nucleotide sequence and functional map of pC194, a plasmid that specified inducible chlormaphenicol resistance. J Bacteriol 150:815–825
Iordanescu S (1975) Recombinant plasmid obtained from two different, compatible Staphylococcal plasmids. J Bacteriol 124:597–601
Iordanescu S, Surdeanu M (1983) Isolation and complementation of temperature-sensitive replication mutants of Staphylococcus aureus plasmid pC194. Mol Gen Genet 191:201–206
Khan S, Carleton S, Novick R (1981) Replication of plasmid pT181 DNA in vitro: Requirement for a plasmid coded product. Proc Natl Acad Sci USA 78:4902–4906
Koepsel RR, Murray RW, Rosenblum WR, Khan S (1985) The replication initiator protein of plasmid pT181 has sequence specific endonuclease and topoisomerase-like activities. Proc Natl Acad Sci USA 82:6845–6849
Kumar CC, Novick RP (1985) Plasmid pT181 replication is regulated by two countertranscripts. Proc Natl Acad Sci USA 227:680–685
Light J, Molin S (1983) Post-transcriptional control of expression of the repA gene of plasmid R1 mediated by a small RNA molecule. EMBO J 2:93–98
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Miller JF (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, New York
Mizuno T, Chou MY, Inouye M (1984) A unique mechanism regulating gene expression: Translational inhibition by a complementary RNA transcrip (micRNA). Proc Natl Acad Sci USA 81:1966–1970
Novick RP, Projan S, Kumar CCh, Carleton S, Gruss A, Highlander SK, Kornblum J (1985) pp 290–320. In: Helinski DR, Cohen SN, Clewell DB, Jackson DA, Hollander A (eds) Plasmids in Bacteria. Plenum Press, New York, London
Rottländer E, Trautner TA (1970) Genetic and transfection studies with B. subtilis phage SP50. I. Phage mutants with restricted growth on B. subtilis strain 168. Mol Gen Genet 108: 47–60
Rownd RH, Womble DD, Dong X-N, Luckov VA, Wu RP (1985) In: Helinski DR, Cohen SN, Clewell DB, Jackson DA, Hollander A (eds) Plasmids in Bacteria, Plenum Press, New York, London, pp 335–354
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Scott JR (1984) Regulation of plasmid replication. Microbiol Rew 48:1823
Shivakumar AG, Hahn J, Dubnau D (1979) Studies on the synthesis of plasmid-encoded proteins and their control in Bacillus subtilis minicells. Plasmid 2:279–289
Soberon X, Covarrubias L, Bolivar F (1980) Construction and characterization of new cloning vehicles: IV Deletion derivatives of pBR322 and pBR325 Gene 9:287–305
Studier FW, Moffatt BA (1986) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130
Tomizawa JI (1984) Control of ColE1 plasmid replication: the process of binding of RNA I to the primer transcript. Cell 38:861–870
Vieira J, Messing J (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268
Viret J-F, Alonso JC (1987) Genetion of Linear multigenome-length plasmid molecules in Bacillus subtilis. Nucleic Acids Res 15:6349–6367
Zuker M, Stiegler P (1981) Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res 9:133–148
Author information
Authors and Affiliations
Additional information
Communicated by W. Goebel
Rights and permissions
About this article
Cite this article
Alonso, J.C., Tailor, R.H. Initiation of plasmid pC194 replication and its control in Bacillus subtilis . Mol Gen Genet 210, 476–484 (1987). https://doi.org/10.1007/BF00327200
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00327200