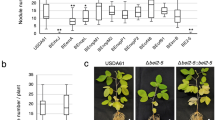
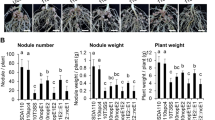
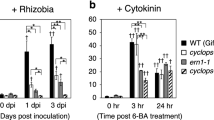
Summary
Rhizobium and Bradyrhizobium bacteria gain intercellular entry into roots of the non-legume Parasponia andersonii by stimulating localized sites of cell division which disrupt the epidermis. Infection threads are then initiated from intercellular colonies within the cortex. Infection via the information of infection threads within curled root hairs, which commonly occurs in legumes, was not observed in Parasponia. The conserved nodulation genes nodABC, necded for the curling of legume root hairs, were not essential for the initiation of infection, however, these genes were required for Parasponia prenodule development. In contrast, the nodD gene of Rhizobium strain NGR234 was essential for the initiation of infection. In addition, successful infection required not only nodD but a region of the NGR234 symbiotic plasmid which is not needed for the nodulation of legumes. Agrobacterium tumefaciens carrying this Parasponia specific region, as well as legume nod genes, was able to form nodules on Parasponia which reached an advanced stage of development.
Similar content being viewed by others
References
Bender GL, Rolfe BG (1985) A rapid plant assay for the Parasponia-Rhizobium symbiosis. Plant Science 38:135–140
Bender GL, Nayudu M, Goydych W, Rolfe BG (1987) Early infection events in the nodulation of the non-legume Parasponia andersonii by Bradyrhizobium. Plant Sci 51:285–293
Bolivar F, Rodriguez R, Greene PJ, Betlach M, Heyneker HL, Boyer HW, Crosa J, Falkow S (1977) Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113
Djordjevic MA, Schofield PR, Ridge RW, Morrison NA, Bassam BJ, Plazinski J, Watson JM, Rolfe BG (1985a) Rhizobium nodulation genes involved in root hair curling (Hac) are functinally conserved. Plant Mol Biol 4:147–160
Djordjevic MA, Schofield PR, Rolfe BG (1985b) Tn5 mutagenesis of Rhizobium trifolii host-specific nodulation genes results in mutants with altered host-range ability. Mol Gen Genet 200:463–471
Downie JA, Knight CD, Johnston AWB, Rossen L (1985) Identification of genes and gene products involved in the nodulation of peas by Rhizobium leguminosarum. Mol Gen Genet 198:255–262
Egelhoff TT, Fisher RF, Jacobs TW, Mulligan JT, Long SR (1985) Nucleotide sequence of Rhizobium meliloti 1021 nodulation genes: nodD is read divergently from nodABC. DNA 4:241–248
Fisher RF, Tu JK, Long SR (1985) Conserved nodulation genes in Rhizobium meliloti and Rhizobium trifolii. Appl Environ Microbiol 49:1432–1435
Foster RC, Rovira AD, Cock TW (1983) Ultrastructure of the Root-Soil Interface. The American Phytopathological Society, St. Paul, Minnesota, USA
Hirsch AM, Drake D, Jacobs TW, Long SR (1985) Nodules are induced on alfalfa roots by Agrobacterium tumefaciens and Rhizobium trifolii containing small segments of the Rhizobium meliloti nodulation region. J Bacteriol 161:223–230
Hooykaas PJJ, van Brussel AAN, den Dulk-Ras H, van Slogteren GMS, Schilperoort RA (1981) Sym plasmid of Rhizobium trifolii expressed in different rhizobial species and Agrobacterium tumefaciens. Nature 291:351–353
Kondorosi E, Banfalvi Z, Kondorosi A (1984) Physical and genetic analysis of a symbiotic region of Rhizobium meliloti: identification of nodulation genes. Mol Gen Genet 193:445–452
Kondorosi A, Kondorosi E, Horvath B, Gottfert M, Bachem C, Rodriguez-Quinones F, Banfalvi Z, Putnoky P, Gyorgypal Z, John M, Schmidt J, Schell J (1987) Common and host specific nodulation genes in Rhizobium meliloti and their conservation in other rhizobia. In: Verma DPS, Brisson N (eds) Molecular Genetics of Plant-Microbe Interactions. Martinus Nijhoff, The Netherlands, pp 217–222
Lancelle SA, Torrey JG (1984) Early development of Rhizobium-induced root nodules of Parasponia rigida. I. Infection and early nodule initiation. Protoplasma 123:26–37
Lancelle SA, Torrey JG (1985) Early development of Rhizobium-induced root nodules of Parasponia rigida. II. Nodule morphogenesis and symbiotic development. Can J Bot 63:25–35
Marvel DJ, Torrey JG, Ausubel FM (1987) Rhizobium symbiotic genes required for nodulation of legume and nonlegume hosts. Proc Natl Acad Sci USA 84:1319–1323
Morrison NA (1984) Genetic analysis of nodulation and nitrogen fixation in the broad host-range Rhizobium strain NGR234. PhD Thesis, Australian National University, Canberra, Australia
Morrison NA, Cen YH, Trinick MJ, Shine J, Rolfe BG (1983) Heat-curing of a Sym plasmid in a fast-growing Rhizobium sp. that is able to nodulate legumes and the non-legume Parasponia sp. J Bacteriol 153:527–531
Morrison NA, Cen YH, Chen HC, Plazinski J, Ridge R, Rolfe BG (1984) Mobilization of a Sym plasmid from a fast-growing cowpea Rhizobium strain. J Bacteriol 160:483–487
Nayudu M, Rolfe BG (1987) Analysis of R-primes demonstrates that genes for broad host range nodulation of Rhizobium strain NGR234 are dispersed on the Sym plasmid. Mol Gen Genet 206:326–337
Plazinski J, Cen YH, Rolfe BG (1985) General method for the identification of plasmid species in fast-growing soil microorganisms. Appl Environ Microbiol 48:1001–1003
Redmond JW, Batley M, Innes RW, Kuempel PL, Djordjevic MA, Rolfe BG (1986) Flavones induce expression of the nodulation genes in Rhizobium. In: Lugtenberg B (ed) Recognition in Microbe-Plant Symbiotic and Pathogenic Interactions. Springer. Berlin, pp 115–122
Rolfe BG, Gresshoff PM, Shine J (1980) Rapid screening for symbiotic mutants of Rhizobium and white clover. Plant Sci Lett 19:277–284
Rolfe BG, Scott KF, Schofield PR, Watson JM, Plazinski J, Djordjevic MA (1985) Genetic and molecular analysis of host range and nodulation genes in Rhizobium trifolii and Parasponia Rhizobium strain ANU289. In: Szalay AA, Legocki RP (eds) Advances in the molecular genetics of the bacteria-plant interaction. Media services, Cornell University, Ithaca, New York, pp 43–48
Rolfe BG, Redmond JW, Batley M, Chen H, Djordjevic SP, Ridge RW, Bassam BJ, Sargent CL, Dazzo FB, Djordjevic MA (1986) Intercellular communication and recognition in the Thizobium-legume symbiosis. In: Lugtenberg B (ed) Recognition in Microbe-Plant Symbiotic and Pathogenic Interactions. Springer, Berlin, pp 39–54
Rossen L, Johnston AWB, Downie JA (1984) DNA sequence of the Rhizobium leguminosarum nodulation genes nodAB and C required for root hair curling. Nucleic Acids Res 12:9497–9508
Scott KF, Saad M, Price GD, Gresshoff PM, Kane H, Kaw YC (1987) Conserved nodulation genes are obligatory for nonlegume nodulation. In: Verma DPS, Brisson N (eds) Molecular Genetics of Plant-Microbe Interactions. Martinus Nijhoff, The Netherlands, pp 238–240
Sinclair MI, Holloway BW (1982) A chromosmally located transposon in Pseudomonas aeruginosa. J Bacteriol 151:569–579
Thomasshow MF, Panagopoulos CG, Gordon MP, Nester EW (1980) Host range of Agrobacterium tumefaciens is determined by the Ti-plasmid. Nature 283:794–796
Torok I, Kondorosi E, Stepkowski T, Posfai J, Kondorosi A (1984) Nucleotide sequence of Rhizobium meliloti nodulation genes. Nucleic Acids Res 12:9509–9524
Trinick MJ (1980a) Relationship amongst the fast-growing rhizobia of Lablab purpureus, Leucaena leucocephala, Mimosa spp., Acacia farnesiana and Sesbania grandiflora and their affinities with other rhizobial groups. J Appl Bacteriol 49:39–53
Trinick MJ (1980b) Growth of Parasponia in agar tube culture and symbiotic effectiveness of isolates from Parasponia spp. New Phytol 85:37–45
Trinick MJ, Galbraith J (1980) The Rhizobium requirements of the non-legume Parasponia in relation to the cross-inoculation group concept of legumes. New Phytol 86:17–26
Turgeon BG, Bauer WD (1982) Early events in the infection of soybean by Rhizobium japonicum. Time course and cytology of the initial infection process. Can J Bot 60:152–161
Turgeon BG, Bauer WD (1985) Ultrastructure of infection-thread development during the infection of soybean by Rhizobium japonicum. Planta 163:328–349
Author information
Authors and Affiliations
Additional information
Communicated by J. Schell
Rights and permissions
About this article
Cite this article
Bender, G.L., Goydych, W., Rolfe, B.G. et al. The role of Rhizobium conserved and host specific nodulation genes in the infection of the non-legume Parasponia andersonii . Molec Gen Genet 210, 299–306 (1987). https://doi.org/10.1007/BF00325698
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00325698