Summary
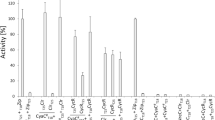
Various truncated CYR1 genes of Saccharomyces cerevisiae were fused to efficient promoters and expressed in Escherichia coli and S. cerevisiae cells with or without the RAS genes. The catalytic domain of adenylate cyclase encoded by the 3′-terminal 1.3 kb region of the open reading frame of the CYR1 gene produced cyclic AMP, irrespective of the presence of RAS genes. The product of the 3′-terminal 2.1 kb region of CYR1 showed guanine nucleotidedependent adenylate cyclase activity and produced a large amount of cAMP in the presence of the RAS gene. Thus, the domain encoded by the 0.8 kb region adjacent to the catalytic domain is associated with the regulatory function of the RAS products. The cyr1 RAS1 RAS2 cells carrying the 3′-terminal 1.3 kb region of CYR1 were unable to respond to environmental signals such as sulfur starvation and temperature shift, but the cyr1 cells carrying the 2.1 kb region and at least one RAS gene were able to respond to these signals. The environmental signals may be transferred to the adenylate cyclase system through the RAS products.
Similar content being viewed by others
References
Broach JR, Strathern JN, Hicks JB (1979) Transformation in yeast: Development of a hybrid cloning vector and isolation of the CAN1 gene. Gene 8:121–133
Broek D, Samiy N, Fasano O, Fujiyama A, Tamanoi F, Northup J, Wigler M (1985) Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell 41:763–769
Casperson GF, Walker N, Brasier AR, Bourne HR (1983) A guanine nucleotide-sensitive adenylate cyclase in the yeast Saccharomyces cerevisiae. J Biol Chem 258:7911–7914
Casperson GF, Walker N, Bourne HR (1985) Isolation of the gene encoding adenylate cyclase in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 82:5060–5063
Chang ACY, Cohen SN (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134:1141–1156
Cohen SN, Chang ACY, Hsu L (1972) Nonchromosomal antibiotic resistance in bacteria: Genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci USA 69:2110–2114
DeFeo-Jones D, Scolnick EM, Koller R, Dhar R (1983) ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature 306:707–709
Dhar R, Nieto A, Koller R, DeFeo-Jones D, Scolnick EM (1984) Nucleotide sequence of two ras H-related genes isolated from the yeast Saccharomyces cerevisiae. Nucleic Acids Res 12:3611–3618
Field J, Broek D, Kataoka T, Wigler M (1987) Guanine nucleotide activation of, and competition between, RAS proteins from Saccharomyces cerevisiae. Mol Cell Biol 7:2128–2133
Gilman AG (1970) A protein binding assay for adenosine 3′,5′-cyclic monophosphate. Proc Natl Acad Sci USA 67:305–312
Holland JP, Holland MJ (1979) The primary structure of a glyceraldehyde-3-phosphate dehydrogenase gene from Saccharomyces cerevisiae. J Biol Chem 254:9839–9845
Iida H, Yahara I (1984) Specific early-G1 blocks accompanied with stringent response in Saccharomyces cerevisiae. J Cell Biol 98:1185–1193
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Kataoka T, Broek D, Wigler M (1985) DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell 43:493–505
Legrain M, Wilde MD, Hilger F (1986) Isolation, physical characterization and expression analysis of the Saccharomyces cerevisiae positive regulatory gene PHO4. Nucleic Acids Res 14:3059–3073
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Masson P, Jacquemin JM, Culot M (1984) Molecular cloning of the tsm0185 gene responsible for adenylate cyclase activity in Saccharomyces cerevisiae. Ann Inst Pasteur Microbiol 135:343–351
Masson P, Lenzen G, Jacquemin JM, Danchin A (1986) Yeast adenylate cyclase catalytic domain is carboxy terminal. Curr Genet 10:343–352
Matsumoto K, Uno I, Oshima Y, Ishikawa T (1982) Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci USA 79:2355–2359
Matsumoto K, Uno I, Ishikawa T (1983) Control of cell division in Saccharomyces cerevisiae. Exp Cell Res 146:151–161
Matsumoto K, Uno I, Ishikawa T (1984) Identification of the structural gene and nonsense alleles for adenylate cyclase in Saccharomyces cerevisiae. J Bacteriol 157:277–282
Matsumoto K, Uno I, Ishikawa T (1985) Genetic analysis of the role of cAMP in yeast. Yeast 1:15–24
Nogi Y, Matsumoto K, Toh-e A, Oshima Y (1977) Interaction of super-repressible and dominant constitutive mutations for the synthesis of galactose pathway enzymes in Saccharomyces cerevisiae. Mol Gen Genet 152:137–144
Powers S, Kataoka T, Fassano O, Goldfarb M, Strathern J, Broach J, Wigler M (1984) Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell 36:607–612
Reddy P, Peterkofsky A, McKenney K (1985) Translational efficiency of the Escherichia coli adenylate cyclase gene: Mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci USA 82:5656–5660
Remaut E, Tsao H, Fiers W (1983) Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene 22:103–113
Rothstein RJ (1983) One-step gene disruption in yeast. Methods Enzymol 101:202–211
Roy A, Danchin A (1982) The cya locus of Escherichia coli K12: Organization and gene products. Mol Gen Genet 188:465–471
Shin D-Y, Matsumoto K, Iida H, Uno I, Ishikawa T (1987) Heat shock response of Saccharomyces cerevisiae mutants altered in cyclic AMP-dependent protein phosphorylation. Mol Cell Biol 7:244–250
Stinchcomb DT, Mann C, Davis RW (1982) Centromeric DNA from Saccharomyces cerevisiae. J Mol Biol 158:157–179
Tamanoi F, Walsh M, Kataoka T, Wigler M (1984) A product of yeast RAS2 gene is a guanine nucleotide binding proteins. Proc Natl Acad Sci USA 81:6924–6928
Temeles GL, DeFeo-Jones D, Tatchell K, Ellinger MS, Scolnick EM (1984) Expression and characterization of ras mRNAs from Saccharomyces cerevisiae. Mol Cell Biol 4:2298–2305
Temeles GL, Gibbs JB, D'Alonzo JS, Sigal IS, Scolnick EM (1985) Yeast and mammalian ras proteins have conserved biochemical properties. Nature 313:700–703
Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M (1985) In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40:27–36
Uno I, Nyunoya H, Ishikawa T (1981) Effects of 2-deoxy-d-glucose and quinidine on the fruiting body formation in Coprinus macrorhizus. J Gen Appl Microbiol 27:219–228
Uno I, Mitsuzawa H, Matsumoto K, Tanaka K, Oshima T, Ishikawa T (1985) Reconstruction of the GTP-dependent adenylate cyclase from products of the yeast CYR1 and RAS2 genes in Escherichia coli. Proc Natl Acad Sci USA 82:7855–7859
Zoller MJ, Smith M (1983) Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol 100:468–500
Author information
Authors and Affiliations
Additional information
Communicated by K. Isono
Rights and permissions
About this article
Cite this article
Uno, I., Mitsuzawa, H., Tanaka, K. et al. Identification of the domain of Saccharomyces cerevisiae adenylate cyclase associated with the regulatory function of RAS products. Molec Gen Genet 210, 187–194 (1987). https://doi.org/10.1007/BF00325683
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00325683