Abstract
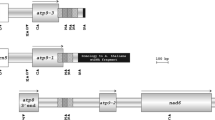
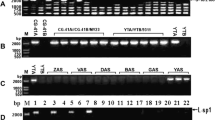
We have characterized the mitochondrial atpA (the alpha subunit of F1-ATPase) gene from male-fertile cytoplasm (cv TK81-0) of sugar beet. The gene is 1518-bp long and encodes a polypeptide of 506 amino acids. The atpA mRNA sequence is modified by three C-to-U RNA editing events, all of which alter the encoded protein sequences. In order to examine the genome organization of the atpA locus in cytoplasmic male-sterile (CMS) sugar beet, atpA-containing clones were isolated from Owen CMS (TK81-MS) and a different source of CMS [I-12CMS(2)] cytoplasm respectively. The sequences of the atpA coding region from TK81-MS and I-12CMS(2) are identical to each other and to the corresponding TK81-0 sequence. However, the TK81-0 and TK81-MS loci diverge completely 47 bp upstream of the initiation codon, resulting in different 5′ transcript termini for the two genes. On the other hand, the point of divergence between the TK81-0 and I-12CMS(2) atpA genes was found to occur after 393 bp 3′ to the TAA stop codon. Our results also show the 3′-flanking sequences of I-12CMS(2) atpA to be present elsewhere in the mitochondrial genomes of TK81-0, TK81-MS and I-12CMS(2), suggesting the possible involvement of these repeated DNA elements in the sequence rearrangements.
Similar content being viewed by others
References
André CP, Levy A, Walbot V (1992) Trends Genet 8:128–132
Bailey-Serres J, Hanson DK, Fox TD, Leaver CJ (1986) Cell 47:567–576
Boutin V, Pannenbecker G, Ecke W, Schewe G, Saumitou-Laprade P, Jean R, Vernet Ph, Michaelis G (1987) Theor Appl Genet 73:625–629
Braun CJ, Levings CS III (1985) Plant Physiol 79:571–577
Brown GG, Auchincloss AH, Covello PS, Gray MW, Menassa R, Singh M (1991) Mol Gen Genet 228:345–355
Covello PS, Gray MW (1989) Nature 341:662–666
Duchenne M, Lejeune B, Fouillard P, Quetier F (1989) Theor Appl Genet 78:633–640
Gualberto JM, Lamattina L, Bonnard G, Weil J-H, Grienenberger J-M (1989) Nature 341:660–662
Halldén C, Bryngelsson T, Bosemark NO (1988) Theor Appl Genet 75:561–568
Hansen BM, Marcker KA (1984) Nucleic Acids Res 12:4747–4756
Hanson MR (1991) Annu Rev Genet 25:461–486
Henikoff S (1984) Gene 28:351–359
Isaac PG, Brennicke A, Dunbar SM, Leaver CJ (1985) Curr Genet 10:321–328
Köhler RK, Lössl A, Zetsche K (1990) Nucleic Acids Res 18:4588
Laver HK, Reynolds SJ, Moneger F, Leaver CJ (1991) Plant J 1:185–193
Leaver CJ, Gray MW (1982) Annu Rev Plant Physiol 33:373–402
Liu AW, Narayanan KK, André CP, Kaleikau EK, Walbot V (1992) Curr Genet 21:507–513
Makaroff CA, Apel IJ, Palmer JD (1990) Plant Mol Biol 15:735–746
Mann V, McIntosh L, Theurer C, Hirschberg J (1989) Theor Appl Genet 78:293–297
Mann V, Ekstein I, Nissen H, Hiser C, McIntosh L, Hirschberg J (1991) Plant Mol Biol 17:559–566
Mikami T, Sugiura M, Kinoshita T (1984) Curr Genet 8:319–322
Mikami T, Kishima Y, Sugiura M, Kinoshita T (1985) Theor Appl Genet 71:166–171
Mikami T, Harada T, Kinoshita T (1986) Curr Genet 10:695–700
Morikami A, Nakamura K (1987) J Biochem 101:967–976
Owen FV (1942) Am J Bot 29:692
Owen FV (1945) J Agric Res 71:423–440
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) Proc Natl Acad Sci USA 74:5463–5467
Saumitou-Laprade P, Pannenbecker G, Boutin-Stadler V, Michaelis G, Vernet P (1991) Theor Appl Genet 81:533–536
Schuster W, Brennicke A (1986) Mol Gen Genet 204:29–35
Schuster W, Hiesel R, Isaac PG, Leaver CJ, Brennicke A (1986) Nucleic Acids Res 14:5943–5954
Schuster W, Ternes R, Knoop V, Hiesel R, Wissinger B, Brennicke A (1991) Curr Genet 20:397–404
Senda M, Harada T, Mikami T, Sugiura M, Kinoshita T (1991) Curr Genet 19:175–181
Siculella L, Palmer JD (1988) Nucleic Acids Res 16:3787–3799
Vera A, Matsubayashi T, Sugiura M (1992) Mol Gen Genet 233:151–156
Author information
Authors and Affiliations
Additional information
Communicated by H. Kössel
Rights and permissions
About this article
Cite this article
Senda, M., Mikami, T. & Kinoshita, T. The sugar beet mitochondrial gene for the ATPase alpha-subunit: sequence, transcription and rearrangements in cytoplasmic male-sterile plants. Curr Genet 24, 164–170 (1993). https://doi.org/10.1007/BF00324681
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00324681