Abstract
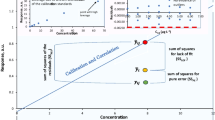
Over the years the authors have developed an empirical formula for the relative standard deviation among laboratories (RSDR, %) as a function of concentration C, expressed as a decimal fraction: RSDR=2(1−0.5log C)≈2C(−0.1505). This formula predicts that starting with a concentration of 100% (pure materials; C=1.00), RSDR will be 2%, increasing by a factor of 2 for each decrease in C of 2 orders of magnitude. It has been a useful guide to acceptable precision in the fields of agricultural, pharmaceutical, and nutritional chemical analysis. It has now been found that this formula is also useful for interpreting the quality of reported analyses of various reference materials available to geochemists. If a ratio is calculated of the found RSDR value to the RSDR value calculated from the formula, an easily interpreted limiting parameter is generated. A ratio >2 provides a rational reference point for interpreting the uncertainty of the analytical portion of the total variability of geochemical concentration estimates. Such a high ratio indicates excessive among-laboratories precision, reflecting large systematic errors on the part of one or more laboratories. The formula quantitates the concept of the transformation of the individual systematic errors of the laboratories into the random error of the group.
Similar content being viewed by others
References
Horwitz W (1988) Pure Appl Chem 60:855–864; (1989) J Asoc Off Anal Chem 72:694–704
Puschel R (1968) Mikrochim Acta 783–801
Horwitz W, Kamps LR, Boyer KW (1980) J Assoc Off Anal Chem 63:1344–1354
Horwitz W (1982) Anal Chem 54:A67-A76
Gladney ES, Roelandts I (1988) Geostand Newsl 12:63–118
Horwitz W, Albert R (1991) ACS Symposium Series 445, American Chemical Society, Washington, DC, pp 50–73
Flanagan FJ (1984) US Geol Surv Bull 1623, Washington, DC
Gladney ES, O'Malley BT, Roelandts I, Gills TE (1987) Standard reference materials: Compilation of elemental concentration data for NBS clinical, biological, geological, and environmental standard reference materials. National Bureau of Standards Special Publication 260-111. Superintendent of Documents, Washington, DC, p 547
Currie LA (1978) Sources of error and the approach to accuracy in analytical chemistry. In: Kolthoff IM, Elving PJ, (eds) Treatise on analytical chemistry, 2nd edn, chap 4. Wiley, New York, pp 122, 124
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Horwitz, W., Albert, R. Precision in analytical measurements: Expected values and consequences in geochemical analyses. Fresenius J Anal Chem 351, 507–513 (1995). https://doi.org/10.1007/BF00322724
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00322724