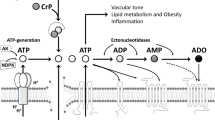
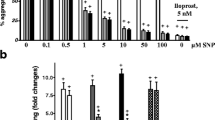
Summary
Previously, the role of adenine nucleotides was thought to be confined to the intracellular space of the cell. Research of the last decades has revealed that nucleotides also occur in the extracellular milieu. This survey deals with extracellular adenine compounds in the blood, focussing on their role as chemical mediators in the haemostatic effect of red cells. Erthrocytes may act as pro-aggregatory cells by at least two chemical mechanisms. Firstly, they can enhance platelet aggregation by releasing adenosine diphosphate (ADP), a well known platelet stimulatory substance. ADP is set free when red cells are stressed mechanically, for instance by shear forces generated in the blood stream; ample experimental evidence supporting this view is summarized. Secondly, erythrocytes efficiently take up extracellular adenosine via their nucleoside transporters, thereby removing a potent inhibitor of platelet function. Extracellular adenosine occurs in the blood stream, either directly released from various tissues or as the end product of extracellular adenine nucleotide metabolism, e.g. after degradation of red cell-born ADP or ATP. Finally, a novel mechanism of action of the antithrombotic drug dipyridamole, which has very recently been put forward, is demonstrated. Dipyridamole inhibits platelet function indirectly by blocking the uptake of extracellular adenosine via the nucleoside transporter of red cells; increased adenosine levels in turn are responsible for the antiaggregatory effect of dipyridamole.
Similar content being viewed by others
References
Aarts PAMM, Bolhuis PA, Sakariassen KS, Heethaar RM, Sixma JJ (1983) Red blood cell size is important for adherence of blood platelets to artery subendothelium. Blood 62: 214–217
Aarts PAMM, Heethaar RM, Sixma JJ (1984) Red blood cell deformability influences platelet-vessel wall interaction in flowing blood. Blood 64: 1228–1233
Abbate R, Favilla S, Boddi M, Costanzo G, Prisco D (1986) Factors influencing platelet aggregation in whole blood. Am J Clin Pathol 86: 91–96
Amer MS, Mayol RF (1973) Studies with phosphodiesterase. III. Two forms of the enzyme from human blood platelets. Biochim Biophys Acta 309: 149–156
Aursnes I, Gjesdal K, Abildgaard U (1981) Platelet aggregation induced by ADP from unsheared erythrocytes at physiological Ca++-concentrations. Br J Haematol 47: 149–152
Balduini CL, Bertolino G, Noris P, Ascari E (1988) The effect of red cells on platelet aggregation: a study with the electronic whole blood aggregometer. Scand J Clin Lab Invest 48: 337–340
Barankiewicz J, Dosch H-M, Cohen A (1988) Extracellular nucleotide catabolism in human B and T lymphocytes. The source of adenosine production. J Biol Chem 263: 7094–7098
Bergamini C, Grazi E (1980) Human platelets 5′-nucleotidase: a cell membrane ectoenzyme with a possible regulatory role in the aggregation reaction. Ital J Biochem 29: 273–288
Bergquist D, Arfors K-E (1980) Haemostatic platelet plug formation in the isolated rabbit mensenteric preparation — an analysis of red blood cell participation. Thromb Haemost 44: 6–8
Boeynaems JM, Galand N (1983) Stimulation of vascular prostacyclin synthesis by extracellular ADP and ATP. Biochem Biophys Res Commun 112: 290–296
Born GVR (1962) Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 194: 927–929
Born GVR (1985) Adenosine diphosphate as a mediator of platelet aggregation in vivo: an editorial view. Circulation 72: 741–746
Born GVR, Bergquist D, Arfors K-E (1976) Evidence for inhibition of platelet activation in blood by a drug effect on erythrocytes. Nature 259: 233–235
Born GVR, Cross MJ (1963) Inhibition of the aggregation of blood platelets by substances related to adenosine diphosphate. J Physiol 166: 29P-30P
Born GVR, Görög P, Kratzer MAA (1981) Aggregation of platelets in damaged vessels. Phil Trans R Soc Lond B 294: 241–250
Born GVR, Wehmeier A (1979) Inhibition of platelet thrombus formation by chlorpromazine acting to diminish haemolysis. Nature 282: 212–213
Cameron DJ (1984) Inhibition of macrophage mediated cytotoxicity by exogenous adenosine 5′-triphosphate. J Clin Lab Immunol 15: 215–218
Cameron DJ (1985) Inhibition of neutrophil-mediated cytotoxicity by exogenous adenosine 5′-triphosphate. Clin Immunol Immunopathol 37: 230–235
Cardinal DC, Flower RJ (1980) The electronic aggregometer: a novel device for assessing platelet behaviour in blood. J Pharmacol Methods 3: 135–158
Carter AJ, Heptinstall S (1985) Platelet aggregation in whole blood: the role of thromboxane A2 and adenosine diphosphate. Thromb Haemost 54: 612–616
Chambers DA, Salzmann EW, Neri LL (1967) Characterization of “ecto-ATPase” of human blood platelets. Arch Biochem. Biophys 119: 173–178
Clemens MG, Forrester T (1980) Appearance of adenosine triphosphate in the coronary sinus effluent from isolated working rat heart in response to hypoxia. J Physiol 312: 143–158
Cockcroft S, Gomperts BD (1979b) ATP induces nucleotide permeability in rat mast cells. Nature 279: 541–542
Cockcroft S, Gomperts BD (1980) the ATP4-receptor of rat mast cells. Biochem J 188: 789–798
Constantinides P (1966) Plaque fissures in human coronary thrombosis. J Atheroscler Res 6: 1–17
Cooper PH, Stanworth DR (1976) Characterization of calcium-ion-activated adenosine triphosphatase in the plasma membrane of rat mast cells. Biochem J 156: 691–700
Cusack NJ, Pearson JD, Gordon JL (1983) Stereoselectivity of ectonucleotidases on vascular endothelial cells. Biochem J 214: 975–981
Dawicki DD, Agarwal KC, Parks RE Jr. (1985) Role of adenosine uptake and metabolism by blood cells in the antiplatelet actions of dipyridamole, dilazep and nitrobenzylthioinosine. Biochem Pharmacol 34: 3965–3972
Dawicki DD, Agarwal KC, Parks RE Jr. (1986) Potentiation of the antiplatelet action of adenosine in whole blood by dipyridamole or dilazep and the cAMP phosphodiesterase inhibitor, RA 233. Thromb Res 43: 161–175
De Mey JG, Vanhoutte PM (1981) Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol 316: 347–355
De-Pierre JW, Karnovsky ML (1974a) Ecto-enzymes of the guinea pig polymorphonuclear leucocyte. 1. Evidence for an ecto-adenosine monophosphatase, -adenosine triphosphatase and -p-nitrophenyl phosphatase. J Biol Chem 249: 7111–7120
De-Pierre JW, Karnovsky ML (1974b) Ecto-enzymes of the guinea pig polymorphonuclear leucocyte. II. Porperties and suitability as markers for the plasma membrane. J Biol Chem 249: 7121–7129
Dieterle Y, Ody C, Ehrensburger A, Stalder H, Junod AF (1978) Metabolism and uptake of adenosine triphosphate and adenosine by porcine aortic and pulmonary endothelial cells and fibroblasts in culture. Circ Res 42: 869–876
Dosne AM, Legrand C, Bauvois B, Bodevin E, Caen JP (1978) Comparative degradation of adenylnucleotides by cultured endothelial cells and fibroblasts. Biochem Biophys Res Commun 85: 183–189
Drury AN, Szent-Györgi A (1929) The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol 28: 213–237
Duke WW (1910) The relation of blood platelets to hemorrhagic disease. J Am Med Assoc 55: 1185–1192
Eliasson R, Bygdeman S (1969) Effect of dipyridamole and two pyrimido-pyrimidine derivatives on the kinetics of human platelet aggregation and on platelet adhesiveness. Scand J Clin Lab Invest 24: 145–151
El-Moatassim C, Dornand J, Mani J-C (1987) Extracellular ATP increases cytosolic free calcium in thymocytes and initiates blastogenesis of the phorbol 12-myristate 13-acetatetreated medullary population. Biochim Biophys Acta 927: 437–444
Emmons PR, Harrison MGJ, Honour AJ, Mitchell JRA (1965) Effect of a pyrimidopyrimidine derivative on thrombus formation in the rabbit. Nature 208: 255–257
Emmons PR, Harrison MGJ, Honour AJ, Mitchell JRA (1965) Effect of dipyridamole on human platelet behaviour. Lancet II: 603–606
FitzGerald GA (1987) Dipyridamole. New Engl J Med 316: 1247–1257
Forrester T, Lind AR (1969) Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol 204: 347–364
Forrester T. Williams CA (1977) Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol 268: 371–390
Freyer DR, Boxer LA, Axtell RA, Todd RF (1988) Stimulation of human neutrophil adhesive properties by adenine nucleotides. J Immunol 141: 580–586
Friedman M, Byers SO (1965) Induction of thrombi upon pre-existing arterial plaques. Am J Pathol 46: 567–575
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376
Gaarder A, Jonsen J, Laland S, Hellem A, Owren PA (1961) Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature 192: 531–532
Gerlach E, Becker BF (eds) (1987) Topics and perspectives in adenosine research. Springer, Berlin Heidelberg New York
Glasgow JG, Schade R, Pitlick FA (1978) Evidence that ADP hydrolysis by human cells is related to thrombogenic potential. Thromb Res 13: 255–266
Goldsmith HL (1971) Red cell motions and wall interactions in tube flow. Fed Proc 30: 1578–1588
Gomperts BD (1984) Manipulation of the cytosolic composition of mast cells: a study of early events in stimulus-secretion coupling. In: Dean RT, Stahl P (eds) Secretory Processes. Butterworths, London, pp 18–37
Greenberg S, Di Virgilio F, Steinberg TH, Silverstein SC (1988) Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem 263: 10337–10343
Gresele P, Arnout J, Deckmyn H, Vermylen J (1986) Mechanism of the antiplatelet action of dipyridamole in whole blood: modulation of adenosine concentration and activity. Thromb Haemost 55: 12–18
Gresele P, Zoja C, Deckmyn H, Arnout J, Vermylen J, Verstraete M (1983) Dipyridamole inhibits platelet aggregation in whole blood. Thromb Haemost 50: 852–856
Haslam RJ, Cusack NJ (1981) Blood platelet receptors for ADP and for adenosine. In: Burnstock G (ed) Purinergic Receptors. Chapman and Hall, London New York, pp 221–285
Hayes LW, Goguen CA, Stevens AL, Magargal WW, Slakey LL (1979) Enzyme activities in endothelial cells and smooth muscle cells from swine aorta. Proc Natl Acad Sci USA 76: 2532–2535
Holmsen H, Rozenberg MC (1968) Adenine nucleotide metabolism of blood platelets. I. Adenosine kinase and nucleotide formation from exogenous adenosine and AMP. Biochim Biophys Acta 155: 326–341
Holmsen H, Weiss HJ (1979) Secretable storage pools in platelets. Ann Rev Med 30: 119–134
Hourani SMO, Cusack NJ (1985) Actions and structure-activity relationships of purines on platelets. In: Stone TW (ed) Purines — Pharmacology and Physiological Roles. Verlag Chemie, Weinheim, pp 163–173
Ireland DM, Mills DCB (1966) Detection and determination of adenosine diphosphate and related substances in plasma. Biochem J 99: 283–296
Kadatz RA (1959) Die pharmakologischen Eigenschaften der neuen koronarerweiternden Substanz 2,6-Bis(diaethanolamino)-4,8-dipiperidino-pyrimido(5,4-d)pyrimidin. Arzneiforschung 9: 39–45
Klabunde RE (1983) Dipyridamole inhibition of adenosine metabolism in human blood. Eur J Pharmacol 93: 21–26
Lewis GP, Lieberman GE, Westwick J (1977) Potentiation of ADPase activity of aortic rings by prostaglandin synthetase and PGE1. Br J Pharmacol 61: 449–450
Lieberman GE, Leake DS, Peters TJ (1982) Subcellular localization of adenosine diphosphatase in cultured pig arterial endothelial cells. Thromb Haemost 47: 249–253
Livio M, Gotti E, Marchesi D, Mecca G, Remuzzi G, de Gaetano G (1982) Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet II: 1013–1015
Lollar P, Owen WG (1981) Active-site-dependent, thrombininduced release of adenine nucleotides from cultured human endothelial cells. Ann N Y Acad Sci 370: 51–56
Lüthje J, Ogilvie A (1988) Catabolism of Ap4A and Ap3A in whole blood. The dinucleotides are long-lived signal molecules in the blood ending up as intracellular ATP in the erythrocytes. Eur J Biochem 173: 241–245
Lüthje J, Schomburg A, Ogilvie A (1988) Demonstration of a novel ecto-enzyme on human erythrocytes, capable of degrading ADP and of inhibiting ADP-induced platelet aggregation. Eur J Biochem 175: 285–289
Lüthje J, Schomburg A, Ogilvie A (1988) Possible antithrombotic function of human erythrocytes: demonstration of an ecto-ADPase (EC 3.6.1.6) capable of inhibiting ADP-induced platelet aggregation. Biol Chem Hoppe-Seyler 369: 869
Medzihradsky F, Cullen EJ, Lin H-L, Bole GG (1980) Drug-sensitive ecto-ATPase in human leucocytes. Biochem Pharmacol 29: 2285–2290
Meyskens FL, Williams HE (1971) Adenosine metabolism in human erythrocytes. Biochim Biophys Acta 240: 170–179
Mills DCB, Smith JB (1971) The influence on platelet aggregation of drugs that affect the accumulation of adenosine 3′:5′-cyclic monophosphate in platelets. Biochem J 121: 185–196
Mustard JF, Moore S, Packham MA, Kinlough-Rathbone RL (1977) Platelets, thrombosis and atherosclerosis. Prog Biochem Pharmacol 13: 312–325
Nachman RL, Ferris B (1974) Binding of adenosine diphosphate by isolated membranes from human platelets. J Biol Chem 249: 704–710
Newby AC (1986) How does dipyridamole elevate extracellular adenosine concentration? Predictions from a three-compartment model of adenosine formation and inactivation. Biochem J 237: 845–851
Novogrodsky A (1972) Concanavalin A stimulation of rat lymphocyte ATPase. Biochim Biophys Acta 266: 343–349
Packham MA, Mustard JF (1977) Clinical pharmacology of platelets. Blood 50: 555–573
Paddle BM, Burnstock G (1974) Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels 11: 110–119
Parker JC, Castranova V, Goldinger JM (1977) Dog red blood cells: Na and K diffusion potentials with extracellular ATP. J Gen Physiol 69: 417–430
Paterson ARP, Harley ER, Cass CE (1984) Inward fluxes of adenosine in erythrocytes and cultured cells measured by a quenched-flow method. Biochem J 224: 1001–1008
Pearson JD, Carleton JS, Gordon JL (1980) Metabolism of adenine nucleotides by ectoenzymes of vascular endothelial and smooth-muscle cells in culture. Biochem J 190: 421–429
Pearson JD, Carleton JS, Hutchings A, Gordon JL (1978) Uptake and metabolism of adenosine by pig aortic endothelial and smooth-muscle cells in culture. Biochem J 170: 265–271
Pearson JD, Coade SB, Cusack NJ (1985) Characterization of ectonucleotidases on vascular smooth-muscle cells. Biochem J 230: 503–507
Pearson JD, Gordon JL (1979) Vascular endothelial and smooth-muscle cells in culture selectively release adenine nucleotides. Nature 281: 384–386
Pearson JD, Slakey LL, Gordon JL (1983) Stimulation of prostaglandin production through purinoceptors on cultured porcine endothelial cells. Biochem J 214: 273–276
Plagemann PGW, Woffendin C (1988) Species differences in sensivity of nucleoside transport in erythrocytes and cultured cells to inhibition by nitrobenzylthioinosine, dipyridamole, dilazep and lidoflazine. Biochim Biophys Acta 969: 1–8
Reimers RC, Sutera SP, Joist JH (1984) Potentation by red blood cells of shear-induced platelet aggregation: relative importance of chemical and physical mechanisms. Blood 64: 1200–1206
Riess H, Braun G, Brehm G, Hiller E (1986) Critical evaluation of platelet aggregation in whole human blood. Am J Clin Pathol 85: 50–56
Saniabadi AR, Lowe GDO, Barbenel JC, Forbes CD (1984) A comparison of spontaneous platelet aggregation in whole blood with platelet rich plasma: additional evidence for the role of ADP. Thromb Haemost 51: 115–118
Saniabadi AR, Lowe GDO, Barbenel JC, Forbes CD (1987) Effect of dipyridamole on spontaneous platelet aggregation in whole blood decreases with the time after venepuncture: evidence for the role of ADP. Thromb Haemost 58: 744–748
Schmid-Schönbein H, Wells R, Goldstone J (1969) Influence of deformability of human red cells upon blood viscosity. Circ Res 25: 131–143
Schmidt A, Ortaldo JR, Herbermann RB (1984) Inhibition of human natural killer cell reactivity by exogenous adenosine 5′-triphosphate. J Immunol 132: 146–150
Schrader J, Berne RM, Rubio R (1972) Uptake and metabolism of adenosine by human erythrocyte ghosts. Am J Physiol 223: 159–166
Small M, Lowe GDO, Cameron E, Forbes CD (1983) Contribution of the haematocrit to the bleeding time. Haemostasis 13: 379–384
Smith GP, Peters TJ (1981) Sucellular localization and properties of adenosine diphosphatase activity in human polymorphonuclear leukocytes. Biochim Biophys Acta 673: 234–242
Smith GP, Shah T, Webster ADB, Peters TJ (1981) Studies on the kinetic properties and subcellular localization of adenosine diphosphatase activity in human peripheral blood lymphocytes. Clin Exp Immunol 46: 321–326
Smolen JE, Weissmann G (1978) Mg2+-ATPase as a membrane ecto-enzyme of human granulocytes. Biochim Biophys Acta 512: 525–538
Sollevi A, Östergren J, Hjemdahl P, Fredholm BB, Fagrell B (1982) The effect of dipyridamole on plasma adenosine levels and skin micro-circulation in man. J Clin Chem Clin Biochem 20: 420–421
Stormorken H (1971) Platelets, thrombosis and hemolysis. Fed Proc 30: 1551–1556
Sullivan JM, Harken DE, Gorlin R (1971) Pharmacologic control of thromboembolic complications of cardic-valve replacement. N Engl J Med 284: 1391–1394
Trams EG, Kaufmann H, Burnstock G (1980) A proposal for the role of ecto-enzymes and adenylates in traumatic shock. J Theor Biol 87: 609–621
Turcáni P, Turcáni M, Bartko D (1982) The effect of β-adrenoceptor blocking drugs on ecto-ATPase activity of rat blood platelets. Biochem Pharmacol 31: 3122–3124
Turitto VT, Baumgartner HR (1975) Platelet interaction with subendothelium in a perfusion system: physical role of red blood cells. Microvasc Res 9: 335–344
Turitto VT, Weiss HJ (1980) Red blood cells; their dual role in thrombus formation. Science 207: 541–543
Wilson PD, Liebermann GE, Peters TJ (1982) Ultrastructural localization of adenosine diphosphatase activity in cultured aortic cells. Histochem J 14: 215–219
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lüthje, J. Extracellular adenine compounds, red blood cells and haemostasis: facts and hypotheses. Blut 59, 367–374 (1989). https://doi.org/10.1007/BF00321207
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00321207