Summary
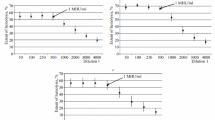
The capacity of immunoglobulin for intravenous application (IgG-IV) to interact with Fc receptors of human monocytes and macrophages was tested by quantifying the inhibition of phagocytosis of IgG-sensitized erythrocytes. To this end a spectrometric phagocytosis test has been used. When compared with IgG for i.m. use (IgG-IM), all IgG-IV had reduced activity. This reduction was related, in part, to the reduced amount of IgG dimers and polymers in IgG-IV. On a weight basis dimeric IgG and polymeric IgG exerted 6-fold and 14-fold higher activity, respectively, than monomeric IgG. When this difference was corrected for, chemically modified IgG-IV still had significantly reduced inhibitory activity; DEAE-Sephadex-treated IgG and acid-treated IgG had an activity similar to IgG-IM, and PEG-treated IgG showed a slightly reduced activity. Pepsin-treated IgG was >100-fold less active than IgG-IM. The reactivity of IgG-IV with monocyte and macrophage Fc receptors was closely correlated. The most conspicuous differences found were related to the concentration at which IgG was used. Thus, β-propiolactone-treated IgG and plasmin-treated IgG were found to have significantly reduced activity at concentrations >20 μg/ml, but almost normal activity when used at lower concentrations.
Similar content being viewed by others
References
Alexander MD, Andrews JA, Leslie RGQ, Wood HW (1980) The binding of human and guinea pig IgG subclasses to homologous macrophage and monocyte receptors. Immunology 35: 115–123
Bøyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest 21 (Suppl 97): 77–80
Burton DR (1985) Immunoglobulin G: functional sites. Mol Immunol 22: 161–206
McCool D, Birshtein BK, Painter RH (1985) Structural requirements of immunoglobulin G for binding to the Fc γ-receptors of the human tumor cell lines U 937, HL-60, ML-1, and K 562. J Immunol 135: 1975–1980
Fleit HB, Wright SD, Unkeless JC (1982) Human Fc γ-receptor distribution and structure. Proc Natl Acad Sci USA 79: 3275–3279
Gross B, Hässig AA, Lüscher EF, Nydegger UE (1983) Monomeric IgG preparations for intravenous use inhibit platelet stimulation by polymeric IgG. Br J Haematol 53: 289–299
Jungi TW (1985) A rapid and sensitive method allowing photometric determination of erythrophagocytosis by mononuclear phagocytes. J Immunol Methods 82: 1451–153
Jungi TW, Barandun S (1985) Estimation of the degree of opsonisation of homologous erythrocytes by IgG for intravenous and intramuscular use. Vox Sang 49: 9–19
Jungi TW, Santer M, Lerch PG, Barandun S (1986) The effect of various treatments of gammaglobulin (IgG) for achieving tolerance on the capacity to interact with human monocyte Fc receptors. Vox Sang 46: in press
Karas SP, Rosse WF, Kurlander JR (1982) Characterization of the IgG-Fc receptor on human platelets. Blood 69: 1277–1282
Kistler P, Nitschmann H (1962) Large scale production of human plasma fractions. Vox Sang 7: 414–424
Law DTS, Painter RH (1986) An examination of the structural and biological properties of three intravenous immunoglobulin preparations. Mol Immunol 23: 331–338
Liehl E, Amerding D, Bockman J, Mayer P, Cooper P (1981) Nonspecific effector functions in various immunoglobulin preparations for I.V. use. In: Nydegger UE (ed) Immunohemotherapy — a guide to immunoglobulin prophylaxis and therapy. Academic Press, New York and London, pp 131–140
Nakagawara A, Nathan CF (1983) A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods 56: 261–268
Pollack M (1983) Antibody activity against Pseudomonas aeruginosa in immune globulins prepared for intravenous use in humans. J Infect Dis 147: 1090–1098
Römer J, Morgenthaler JJ, Scherz R, Skvaril F (1982) Characterisation of various immunoglobulin preparations for intravenous application. I. Protein composition and antibody content. Vox Sang 42: 62–73
Skvaril F, Roth-Wicky B, Barandun S (1980) IgG subclasses in human γ-globulin preparations for intravenous use and their reactivity with Staphylococcus protein A. Vox Sang 38: 147–155
Van Furth R, Leijh PCJ, Klein F (1984) Correlation between opsonic activity for various microorganisms and composition of gammaglobulin preparations for intravenous use. J Infect Dis 149: 511–517
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jungi, T.W., Eiholzer, J., Lerch, P.G. et al. The capacity of various types of immunoglobulin for intravenous use to interact with Fc receptors of human monocytes and macrophages. Blut 53, 321–332 (1986). https://doi.org/10.1007/BF00320892
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00320892