Summary
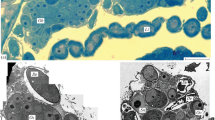
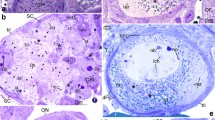
The structure of follicular layer of growing and atretic follicles in the ovary of the domestic goose, was studied by electron microscopy. In small follicles, the wall is lined with a narrow layer of tightly packed small, cuboidal cells separated from the thecal tissue by the basal lamina. During growth, they transform into tall, columnar cells arranged in a single row. The cells display several peculiar ultrastructural features. First, annulate lamellae are commonly observed. Second, cytoplasmic dense-cored granules accumulate in close association with fenestrated cisternae and networks of tubuli derived from the RER. They consist of spheres and strands of amorphous substance of unknown origin. Third, the cells contain many transosomes, a unique organelle of the avian follicle cell consisting of a dense plaque associated with ribosome-like particles. The mature forms of transosomes are located at the tips of lateral and apical cell projections, while bodies thought to be their precursors, are found in the apical cytoplasm. In follicles larger than 8 mm in diameter, most of the transosomes and their precursors have disappeared. Follicular atresia occurs in all of the size-classes of follicles investigated. A loss of transosomes (in follicles up to 8 mm in diameter) and an accumulation of lipid droplets are the first atretic events detectable by electron microscopy. Morphologic features, including deep nuclear indentations, accumulation of lipid droplets frequently encireled by membrane whorls, dilation and disintegration of RER cisterns, swelling of mitochondria and accumulation of dense irregular masses of unknown origin in the cytoplasm, are taken as evidence for advanced degradation. We conclude that necrosis is the dominant type of cell death of the follicular cells during atresia. However, a small fraction of cells, characterized by dark condensed cytoplasm, seems to die by apoptosis.
Similar content being viewed by others
References
Chieffi G, Botte V (1964) The distribution of some enzymes involved in the steroidogenesis of hen's ovary. Experientia 21:16–17
Clarke PGH (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 181:195–213
Dahl E (1970) Studies on the fine structure of ovarian interstitial tissue. 2. The ultrastructure of the thecal gland of the domestic fowl. Z Zellforsch Mikr Anat 109:195–211
Dahl E (1971) The fine structure of the granulosa cells in the domestic fowl and the rat. Z Zellforsch 119:58–67
Deray A (1978) Les cellules de la granulosa des petits follicules ovariens de Canes: Cane Pékin (Anas platyrhynchos), Barbarie (Cairina moschata) et hybride: Pékin mâle X Barbarie femelle. J Ultrastruct Res 68:118–135
Erpino MJ (1973) Histogenesis of atretic follicles in a seasonally breeding bird. J Morphol 139:239–250
Fawcett DW, Long JA, Jones AL (1969) The ultrastructure of endocrine glands. Recent Progr Hormone Res 25:315–368
Forgó V, Afanasiev GD, Péczely P (1988a) Structural and hormonal changes during follicular maturation in the ovary of the domestic goose. Acta Biol Hung 39:403–417
Forgó V, Sass M, Péczely P (1988b) Light microscopic, enzyme biochemical and steroid analytical investigations of follicular atresia in the ovary of domestic goose. Acta Biol Hung 39:377–401
Gilbert AB, Hardie MA, Perry MM, Dick HR, Wells JW (1979) Cellular changes in the granulosa layer of the maturing ovarian follicle of the domestic fowl. British Poultry Sci 21:257–263
Gorbik TA (1975) The ultrastructure of the follicular epithelium in the ovary of the domestic duck during the period of slow growth of the oocyte. Arkh Anat Gistol Embriol 69:30–35 (in Russian)
Gorbik TA (1976) Ultrastructure and dynamics of transosomes of the follicular epithelium of bird ovaries. Arkh Anat Gistol Embriol 70:79–86 (in Russian)
Gorbik TA, Gabaeva NS (1975) Morphological alterations of the follicular epithelium in oogenesis of the duck (Anas boschas domestica) and other birds. Arkh Anat Gistol Embriol 68:16–24 (in Russian)
Gupta SK, Maiti BR (1986) Study of atresia in the ovary during the annual reproductive cycle and nesting cycle of the pied myna. J Morphol 190:235–296
Gupta SK, Gilbert AB, Walker MA (1988) Histological study of follicular atresia in the ovary of the domestic hen (Gallus domesticus). J Reprod Fert 82:219–225
Guraya SS (1976) Morphological and histochemical observations on follicular atresia and interstitial gland tissue in the columbid ovary. Gen Comp Endocrinol 30:534–538
Guraya SS (1989) Ovarian follicles in reptiles and birds. In: Farner DS (ed) Zoophysiology 24. Springer, Berlin Heidelberg New York
Guraya SS, Chalana RK (1976) Histochemical observations on the seasonal fluctuations in the follicular atresia and interstitial gland tissue in the house sparrow ovary. Poultry Sci 55:1881–1885
Hertelendy F, Asem EK (1984) Steroidogenesis in granulosa cells during follicular maturation: evidence for desensitisation-resensitisation during ovulation cycle. J Exp Zool 232:513–520
Kessel RG (1989) The annulate lamellae — from obscurity to spot-light. Electron Microsc Rev 2:257–348
Kovács A (1974) Effects of ACTH and prednisolone on the ultrastructure of interrenal cells of chicken adrenals. Acta Biol Acad Sci Hung 25:255–268
Kudryavtsev IV, Sheina NI, Vasin VI (1982) A comparative electron microscope study of oocytes of the chicken and Coturnix coturnix japonica. Zh Obsch Biol 43:394–398 (in Russian)
Marrone BL, Sebring RJ (1989) Quantitative cytochemistry of 3β-hydroxysteroid dehydrogenase activity in avian granulosa cells during follicular maturation. Biol Reproduction 40:1007–1011
Onagbesan OM, Peddie MJ (1988) Steroid secretion by ovarian cells of the Japanese quail (Coturnix coturnix japonica). Gen Comp Endocrinol 72:272–281
Paulson J, Rosenberg MD (1972) The function and transposition of lining bodies in developing avian oocytes. J Ultrastruct Res 40:25–43
Paulson JL, Rosenberg MD (1974) Formation of lining bodies and oocyte bodies during avian oogenesis. Dev Biol 40:366–371
Perry MM, Gilbert AB, Evans AJ (1978) Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J Anat 125:481–497
Press N (1964) An unusual organelle in avian ovaries. J Ultrastruct Res 10:528–546
Rothwell B, Solomon SE (1977) The ultrastructure of the follicle wall of the domestic fowl during the phase of rapid growth. Br Poult Sci 18:605–610
Sayler A, Dowd AJ, Wolfson A (1970) Influence of photoperiod on the localization of D-3β-hydroxysteroid dehydrogenase in the ovaries of maturing Japanese quail. Gen Comp Endocrinol 15:20–30
Schjeide OA, Galey F, Grellert EA, I-San Lin R, De Vellis J, Mead JF (1970) Macromolecules in oocyte maturation. Biol Reprod Suppl 2:14–43
Schjeide OA, Hanzely L, Holshouser SJ, Briles WE (1974) Production and fates of unique organelles (transosomes) in ovarian follicles of Gallus domesticus under various conditions. Cell Tissue Res 156:47–59
Schjeide OA, Kancheva L, Hanzely L, Briles WE (1975) Production and fates of unique organelles (transosomes) in ovarian follicles of Gallus domesticus under various conditions. II. Cell Tissue Res 163:63–79
Walker NI, Harmon BV, Gobé GC, Kerr JER (1988) Patterns of cell death. Meth Achiev Exp Pathol 13:18–54
Wyburn GM, Aitken RNC, Johnston HS (1965a) The ultrastructure of the ovarian follicle of the domestic fowl. J Anat 99:469–484
Wyburn GM, Johnston HS, Aitken RNC (1965b) Specialised plasma membranes in the preovulatory follicle of the fowl. Z Zellforsch 68:70–79
Wyburn GM, Johnston HS, Aitken RNC (1966) Fate of the granulosa cells in the hen's follicle. Z Zellforsch 72:53–65
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kovasc, J., Forgó, V. & Péczely, P. The fine structure of the follicular cells in growing and atretic ovarian follicles of the domestic goose. Cell Tissue Res 267, 561–569 (1992). https://doi.org/10.1007/BF00319379
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319379